Method for preparing important intermediate 2-(hexadecyloxy carbonyl)-amino-5-methylbenzoic acid of Cetilistat
A technology of methyl benzoic acid and new lixistat, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carbamic acid derivatives, etc., and can solve the problem of low conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The qualitative and quantitative detection method of the reaction substrate and product is: using KromasilC 18 Column (12.5cm×4.6mm×5μm), mobile phase: acetonitrile:acetic acid (100:0.1); UV detection wavelength 221nm; flow rate: 1.0mL / min; column temperature 30°C.
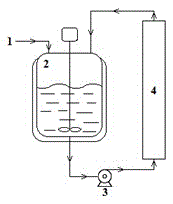
[0021] Put compound II, III, pyridine and solvent dichloromethane into the reaction kettle, wherein the molar ratio of compound II and III is 1:1, vigorously stir the reaction, during the reaction, the reaction feed liquid is pumped into two parallel molecular sieves In addition to the water column. After reacting for 1 hour, the product I was discharged from the bottom of the reaction kettle, heated and dissolved by adding ethyl acetate, and cooled to 10°C for recrystallization for 12 hours. The yield of the obtained product I was greater than 92.57%, and the chemical purity was greater than 99.76%.
Embodiment 2
[0023] Reaction substrate and product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar ratio and each operating parameter are as follows:
[0024] Compound II, III, pyridine and solvent dichloromethane are loaded into the reactor, wherein the molar ratio of compound II and III is 1:1.5, and the reaction is vigorously stirred. During the reaction, the reaction feed liquid is pumped into three parallel packing In addition to the water column of calcium chloride. After 1.5 hours of reaction, the product I was released from the bottom of the reaction kettle, heated and dissolved by adding n-hexane, and cooled to 0°C for 20 hours of recrystallization. The yield of the obtained product I was greater than 95.73%, and the chemical purity was greater than 99.14%.
Embodiment 3
[0026] Reaction substrate and product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar ratio and each operating parameter are as follows:
[0027] Compound II, III, pyridine and solvent dichloromethane are loaded into the reactor, wherein the molar ratio of compound II and III is 1:2, and the reaction is stirred vigorously. During the reaction, the reaction feed liquid is pumped into a single branch filled with anhydrous Sodium sulfate in the water column. After reacting for 2 hours, the product I was released from the bottom of the reactor, added ethanol to heat and dissolve, and cooled to 20°C for recrystallization for 15 hours. The yield of the obtained product I was greater than 93.29%, and the chemical purity was greater than 99.46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com