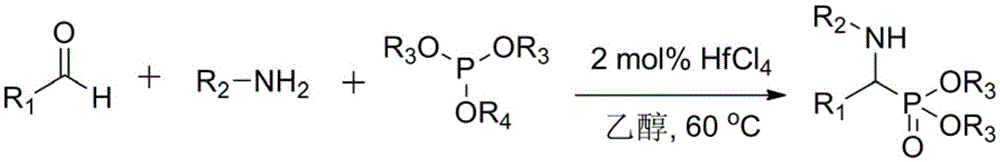
Efficient and novel method for preparing aminophosphonate through catalytic synthesis of hafnium tetrachloride
A hafnium tetrachloride-catalyzed synthesis of aminophosphonate and hafnium tetrachloride technology, which is applied in the field of aminophosphonate synthesis, can solve the problems of poor catalytic effect and no catalytic effect, and achieve fast reaction speed and mild reaction conditions , The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Synthesis of phenyl-alpha-(anilino)methylphosphonic acid dimethyl ester (formula 1):
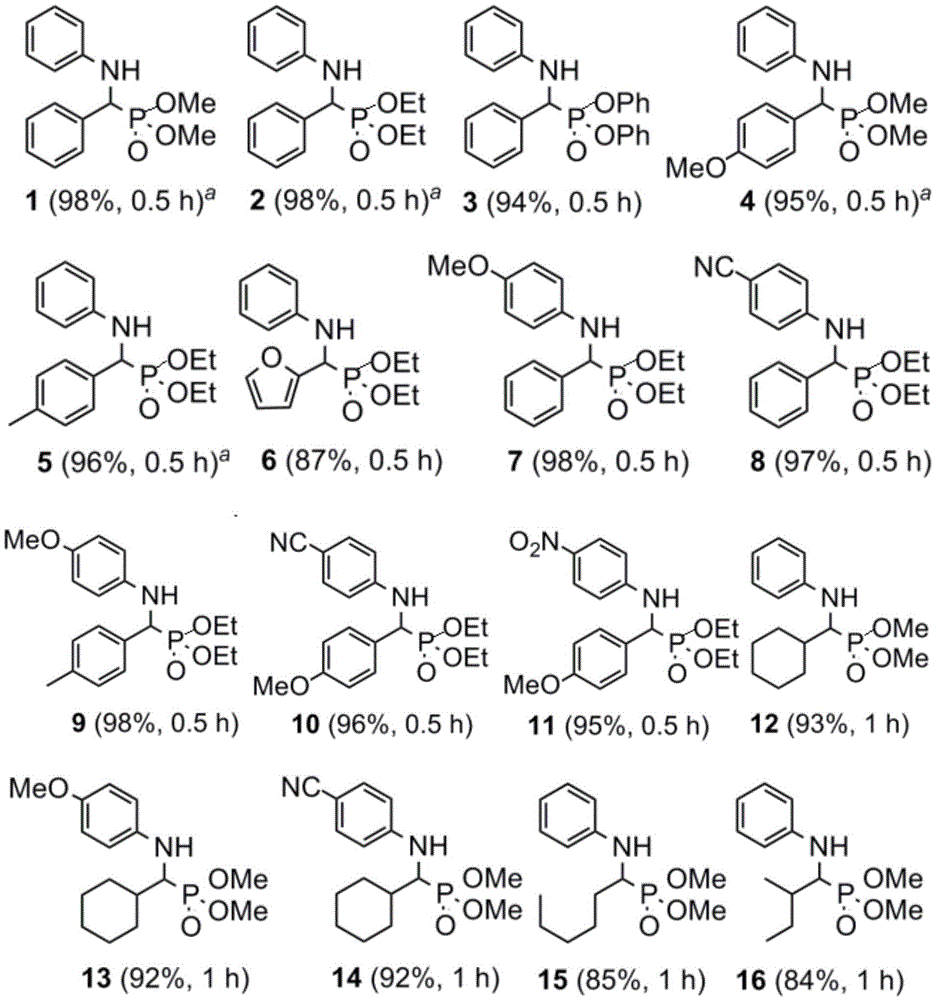
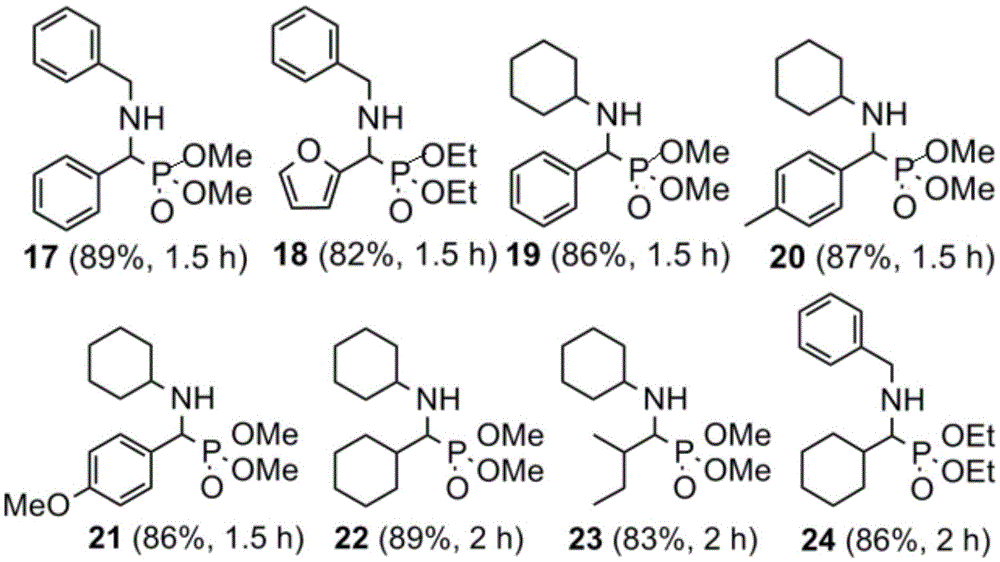
[0018] Benzaldehyde (1.00g, 9.43mmol), aniline (0.88g, 9.43mmol), dimethyl phosphite (1.09g, 9.90mmol) and catalyst hafnium tetrachloride (60mg, 0.19mmol) were dissolved in ethanol (6.7mL ), the concentrations of benzaldehyde and aniline were 1.0mol / L, and reacted at 60°C for 0.5 hours. The reaction solution was concentrated to obtain a crude product, and silica gel column chromatography (petroleum ether / ethyl acetate volume ratio = 1:2) gave 2.69 g of dimethyl phenyl-α-(anilino)methylphosphonate (Formula 1), the yield 98%.
Embodiment 2
[0020] Synthesis of phenyl-α-(4-methylanilino) methylphosphonic acid diethyl ester (formula 7):
[0021] Benzaldehyde (1.00g, 9.43mmol), 4-methylaniline (1.01g, 9.43mmol), triethyl phosphite (1.64g, 9.90mmol) and catalyst hafnium tetrachloride (60mg, 0.19mmol) were dissolved in Ethanol (5.8 mL), the concentrations of benzaldehyde and 4-methylaniline were all 1.0 mol / L, and reacted at 60° C. for 0.5 hour. The reaction solution was concentrated to obtain a crude product, and silica gel column chromatography (petroleum ether / ethyl acetate=1:2) yielded 3.19 g of phenyl-α-(4-methylanilino)methylphosphonic acid diethyl ester (Formula 7), Yield 97%.
Embodiment 3
[0023] Synthesis of cyclohexyl-α-(4-cyanoanilino) methylphosphonic acid dimethyl ester (formula 14):
[0024] Dissolve cyclohexanal (1.00g, 8.93mmol), 4-cyanoaniline (1.05g, 8.93mmol), dimethyl phosphite (1.03g, 9.38mmol) and catalyst hafnium tetrachloride (57mg, 0.18mmol) In ethanol (6.2 mL), the concentrations of cyclohexanal and 4-cyanoaniline are both 1.0 mol / L, and react at 60° C. for 1 hour. The reaction solution was concentrated to obtain a crude product, and silica gel column chromatography (petroleum ether / ethyl acetate=1:2) gave dimethyl cyclohexane-α-(4-cyanoanilino)methylphosphonate (Formula 14) 2.79 g, yield 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com