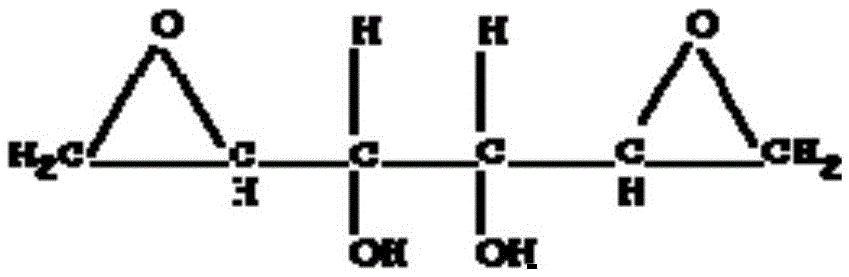
Dianhydrodulcitol lipidosome freeze-dried powder injection and preparation method thereof
A technology of dehydrated dulcitol and freeze-dried powder injection, which is applied in the field of pharmaceutical inventions, can solve the problems of tissue necrosis, difficult remission and treatment of patients, ulcers, etc., and achieves the effects of reducing irritation, improving storage stability, and prolonging circulation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 15kg of lecithin, 15kg of cholesterol, 3kg of dulcitol, dissolve in 95% ethanol, evaporate under reduced pressure to form a film, then add 10L of isotonic phosphate buffer solution with pH=6, the two are emulsified, and then removed under reduced pressure Ethanol, and probe-type ultrasound for 3 minutes to obtain liposomes. 15 kg of sucrose is dissolved in liposomes, filtered, subpackaged in vials, freeze-dried, and sealed with a vacuum plug.
Embodiment 2
[0021] Take 120kg of lecithin, 15kg of cholesterol, 3kg of dulcitol, dissolve in 90% ethanol, evaporate under reduced pressure to form a film, then add 10L of isotonic phosphate buffer solution with pH=6, the two are emulsified, and then removed under reduced pressure Ethanol, and probe-type ultrasound for 3 minutes to obtain liposomes. 120kg of sucrose is dissolved in liposomes, filtered, subpackaged in vials, freeze-dried, and sealed with a vacuum stopper.
Embodiment 3
[0023] Take 30kg of lecithin, 15kg of cholesterol, 3kg of dulcitol, dissolve in 95% ethanol, evaporate under reduced pressure to form a film, then add 10L of isotonic phosphate buffer solution with pH=8, emulsify the two, and remove under reduced pressure Ethanol, and probe-type ultrasound for 3 minutes to obtain liposomes. Dissolving 30kg of sucrose in liposomes, filtering, dispensing into vials, freeze-drying, and sealing with a vacuum plug.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com