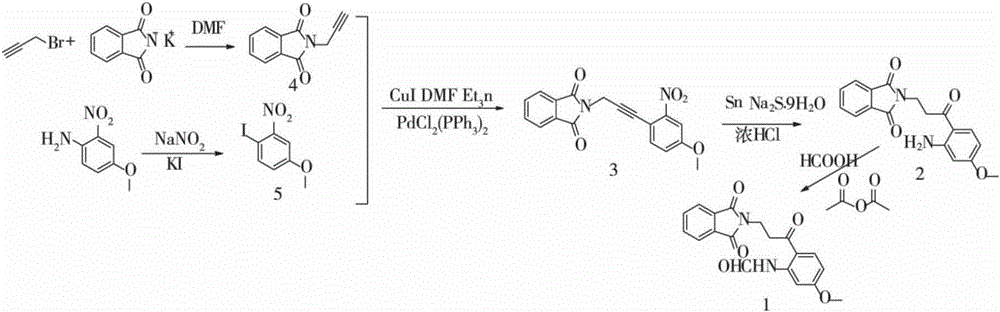
Synthetic method of N-((2-(1,3-dioxo-dihydroisoindol-2-yl)-propionyl)-5-methoxy)formamide
A technology of dioxoisoindoline and synthesis method, applied in the direction of organic chemistry and the like, can solve problems such as unfavorable industrialization promotion, time-consuming, low yield, etc. The effect of activation energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A method for synthesizing N-(2-(1,3-dioxoisoindolin-2-yl)-propionyl)-5-methoxy)formamide, comprising the following steps:
[0036] Step 1: Dissolve 11.9mmol of 2-nitro-4-methoxyaniline in 20mL concentrated sulfuric acid solution (mass concentration is 70%), reduce the temperature of the system to -5℃, set the temperature to 0℃ and the concentration to 2mol 10mL of sodium nitrite solution was quickly added to the vigorously stirred reaction system, and reacted at 0°C for 10 minutes; the reaction system was poured into 5mL of sodium iodide solution with a temperature of 0°C and a concentration of 4mol / L. Continue to react for 30 minutes;
[0037] The filtered residue was washed with 6N hydrochloric acid and water, and the crude product was crystallized in n-hexane to obtain a yellow solid A;
[0038] Step 2: Add 8.4mmol of bromopropyne and 8.4mmol of potassium phthalimide to 10mL of N,N-dimethylformamide, then add 0.2g of solid manganese dioxide, and react at 28°C 1h;
[0039] ...
Embodiment 2
[0047] A method for synthesizing N-(2-(1,3-dioxoisoindolin-2-yl)-propionyl)-5-methoxy)formamide, comprising the following steps:
[0048] The first step: Dissolve 11.9mmol of 2-nitro-4-methoxyaniline in 20mL concentrated sulfuric acid solution (mass concentration of 71%), reduce the system temperature to -5℃, set the temperature to 0℃ and the concentration to 2mol 10mL of sodium nitrite solution was quickly added to the vigorously stirred reaction system, and reacted at 0℃ for 15min; the reaction system was poured into 5mL of sodium iodide solution with a temperature of 0℃ and a concentration of 4mol / L. Continue to react for 30 minutes;
[0049] The filtered residue was washed with 6N hydrochloric acid and water, and the crude product was crystallized in n-hexane to obtain a yellow solid A;
[0050] Step 2: Add 8.4mmol of bromopropyne and 8.4mmol of potassium phthalimide to 10mL of N,N-dimethylformamide, then add 0.3g of solid manganese dioxide, and react at 28°C 2h;
[0051] Add 10...
Embodiment 3
[0059] A method for synthesizing N-(2-(1,3-dioxoisoindolin-2-yl)-propionyl)-5-methoxy)formamide, comprising the following steps:
[0060] Step 1: Dissolve 11.9mmol of 2-nitro-4-methoxyaniline in 20mL concentrated sulfuric acid solution (mass concentration is 70%), reduce the temperature of the system to -5℃, set the temperature to 0℃ and the concentration to 2mol 10mL of sodium nitrite solution was quickly added to the vigorously stirred reaction system, and reacted at 0℃ for 15min; the reaction system was poured into 5mL of sodium iodide solution with a temperature of 0℃ and a concentration of 4mol / L. Continue to react for 30 minutes;
[0061] The filtered residue was washed with 6N hydrochloric acid and water, and the crude product was crystallized in n-hexane to obtain a yellow solid A;
[0062] Step 2: Add 8.4mmol of bromopropyne and 8.4mmol of potassium phthalimide to 10mL of N,N-dimethylformamide, then add 0.2g of solid manganese dioxide, and react at 28°C 2h;
[0063] Add 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com