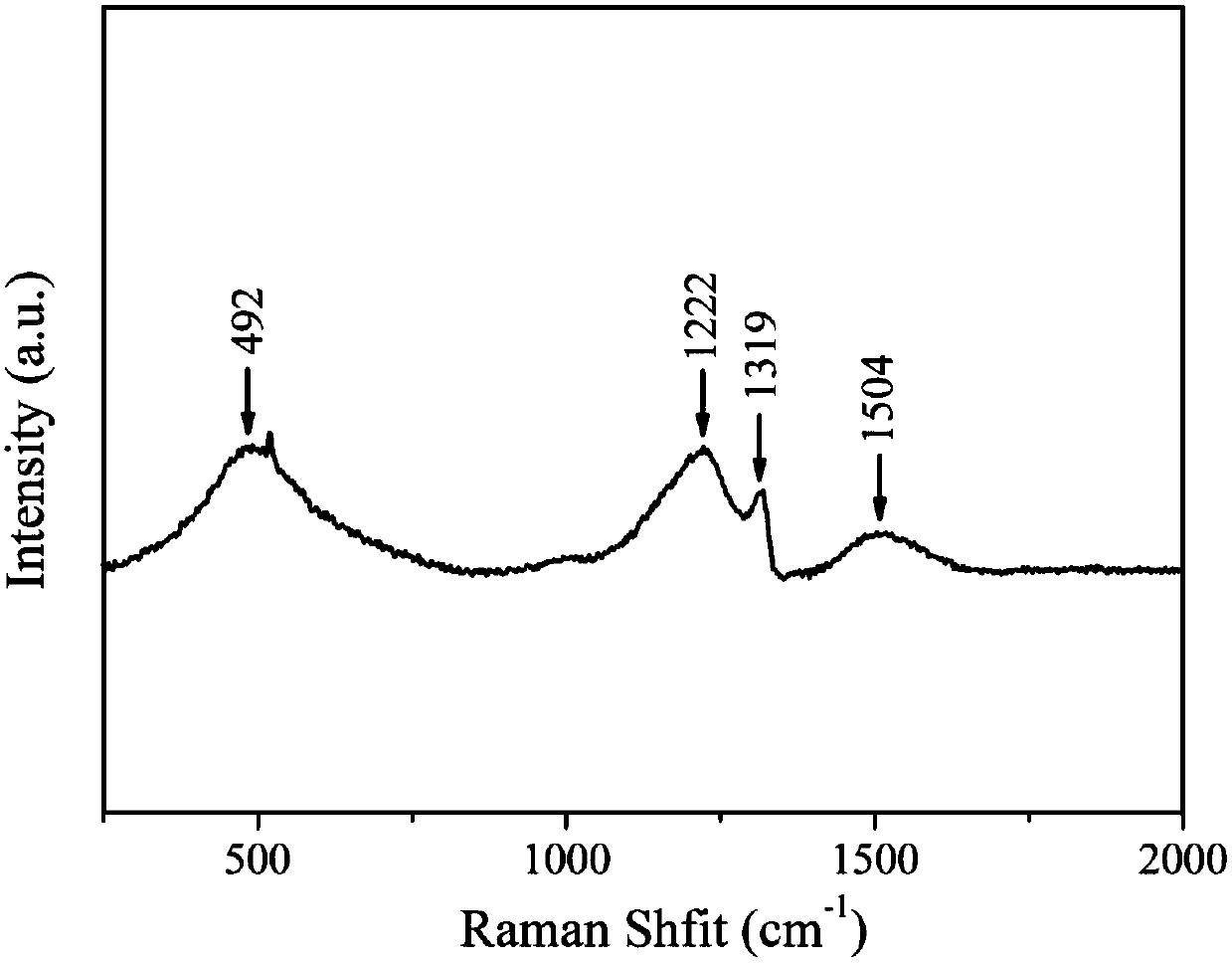
A catalyst for electrochemical reduction of CO2 based on boron-nitrogen co-doped nano-diamond, preparation method and application thereof
A nano-diamond and electrochemical technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of expensive precious metals, scarce catalytic materials, poor product selectivity, etc., and reach the potential window wide, low background current, and high current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
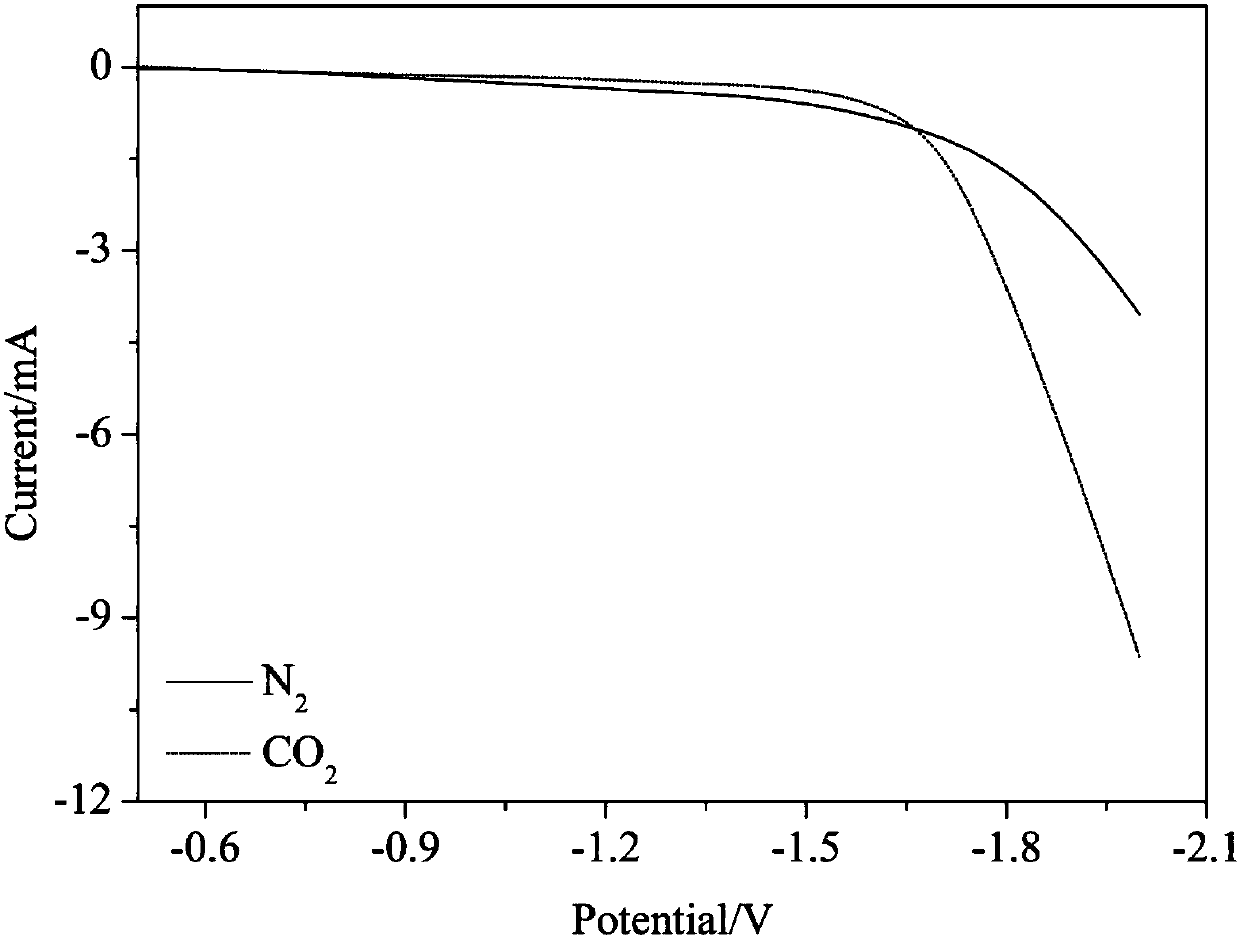
[0018] Example 1, boron and nitrogen co-doped nano-diamond electrochemical reduction of CO 2 active
[0019] A H-type double-cell reactor is used, in which the volume of the single cell is 100mL, the middle of the double cell is separated by a proton exchange membrane, the reactor is sealed, and the cathode chamber and the anode chamber are continuously fed with CO before the reaction. 2 The gas was saturated for 1h, and the gas flow rate was 20 sccm. A three-electrode working system is adopted, boron-nitrogen co-doped nano-diamond is the working electrode, the Pt electrode is the counter electrode, and the Ag / AgCl electrode is the reference electrode. The distance between the working electrode and the Pt electrode is 1.5cm, and the reference electrode is as close as possible to the working electrode. , respectively, in saturated CO 2 and N 2 0.1mol / L NaHCO 3 The linear voltammetry curve is measured in the aqueous solution, the area of the working electrode and the volum...
Embodiment 2
[0022] Example 2, boron and nitrogen co-doped nano-diamond electrochemical reduction of CO 2 stability
[0023] A H-type double-cell reactor is used, in which the single-cell volume is 100mL, and the middle of the double-cell is separated by a proton exchange membrane. The reactor is sealed, and the gas-phase product is collected by an air bag, and the liquid-phase product is detected by gas chromatography. Before the reaction, the cathode chamber and the anode chamber were continuously fed with CO 2 The gas was saturated for 1h, and CO was continuously exposed during the reaction 2 Gas, the gas flow rate is 15 sccm, the cathode chamber is magnetically stirred, and the rotation speed is constant. In the three-electrode working system, boron-nitrogen co-doped nano-diamond is used as the working electrode, the Pt electrode is used as the counter electrode, and the Ag / AgCl electrode is used as the reference electrode. The distance between the working electrode and the Pt electr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com