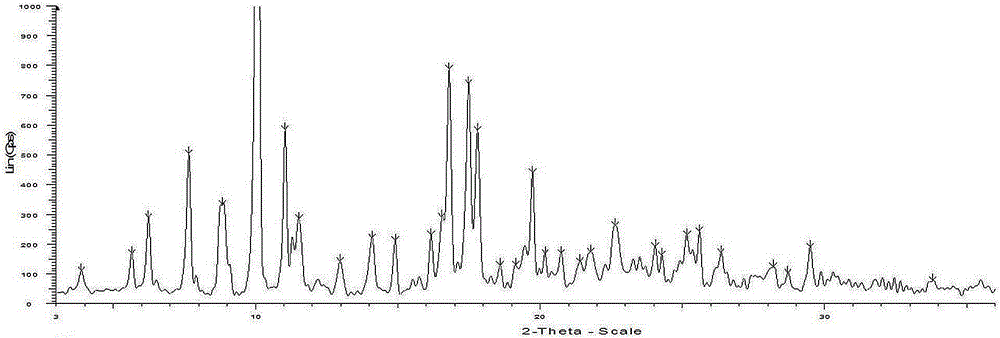
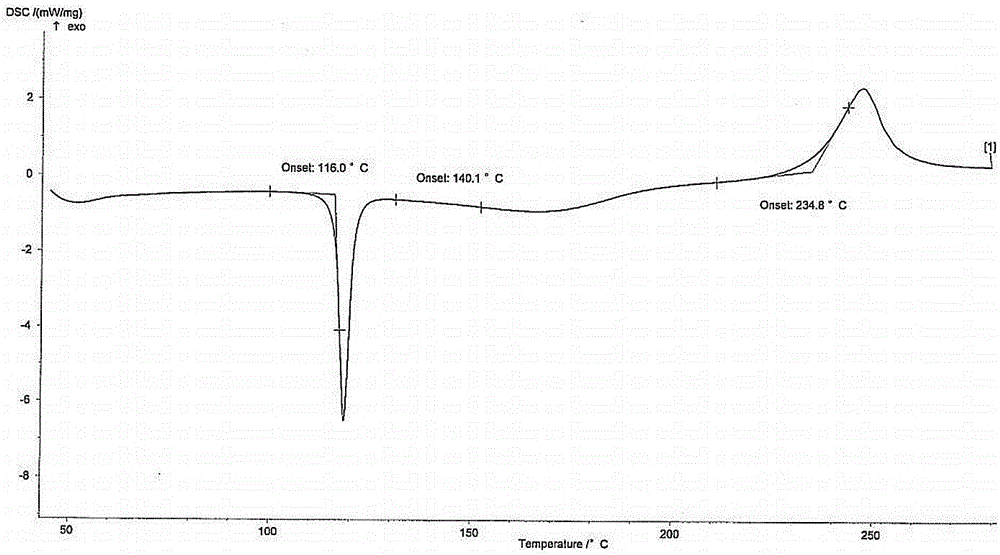
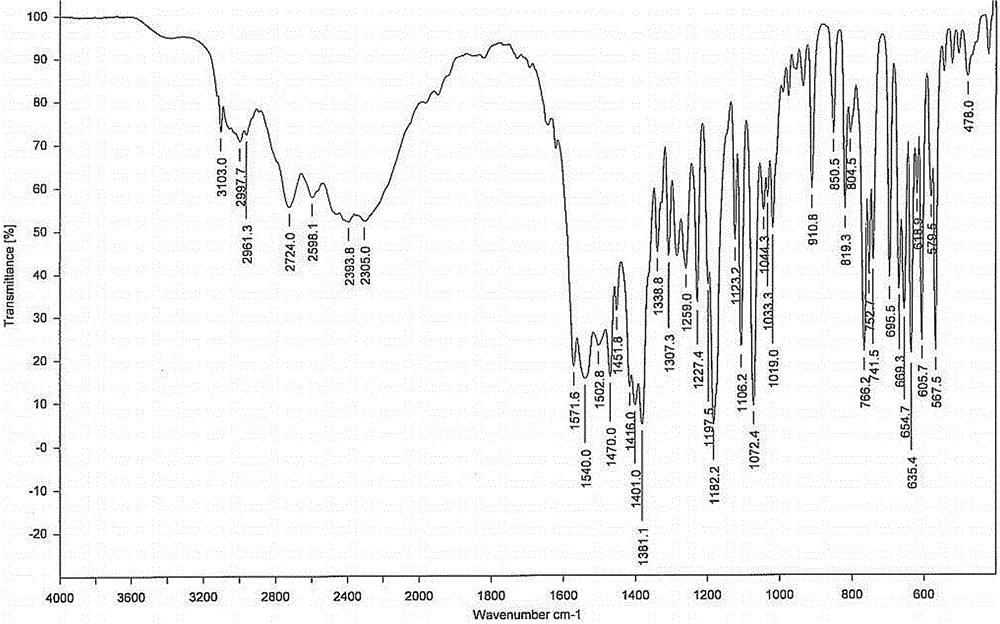
Preparation of pyrrole gastric acid secretion inhibitor compound salt
A technology of pyrrole and organic acid salts, applied in anti-inflammatory agents, digestive system, organic chemistry, etc., can solve the problems of poor water solubility of compounds, limit acid suppression and treat gastric acid-related diseases, and achieve the treatment of gastric acid-related diseases , the effect of meeting the clinical drug demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In a 100 mL one-necked bottle, add 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine (5.0 g, 14.48 mmol) and acetic acid Ethyl ester (25mL) was stirred and dissolved at room temperature (15°C), sulfuric acid (0.5mL, 15.20mmol) was added dropwise to the reaction system, stirred at this temperature for 30 minutes, isopropanol (20mL) was added, and The temperature was lowered to 3°C, and stirred for 30 minutes, a solid precipitated, the solid was filtered out, and washed with ethyl acetate (10mL*2 times), and the obtained solid was dried in a vacuum oven (45°C) for 4 hours to obtain White solid 4.3g, yield 76%.
[0058] 1 HNMR (DMSO- d 6;500MHZ)δ(ppm)8.89(dd, J =4.8, J =1.3,1H),8.72(s,2H),8.56(d, J =2.0,1H),7.92(dd, J =8.1, J =2.1,1H),7.84(s,1H),7.64(m,1H),7.52(m,1H),7.22(m,2H),7.10(m,1H),6.54(d, J =1.5,1H),4.04(s,2H),2.57(s,3H)
[0059] Example 2
Embodiment 2
[0061] In a 100 mL one-necked bottle, add 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine (3.0 g, 6.1 mmol) and acetic acid Ethyl ester (15mL) was stirred and dissolved at room temperature (15°C), phosphoric acid (0.3mL, 4.6mmol, 85%) was added dropwise to the reaction system, stirred at this temperature for 30 minutes, and isopropanol (12mL) was added , and then lowered the temperature to 2°C and stirred for 30 minutes, a solid precipitated out, the solid was filtered out, and washed with ethyl acetate (8mL*2 times), and the obtained solid was dried in a vacuum oven (45°C) for 4 hours , to obtain 3.1 g of off-white solid with a yield of 91.0%.
[0062] 1 HNMR (DMSO- d 6;500MHZ)δ(ppm)8.86(d, J =4.6,1H),8.71(s,2H),8.55(d,J=1.9,1H),7.92(d, J =8.1,1H),7.83(s,1H),7.66(m,1H),7.53(m,1H),7.23(m,2H),7.11(m,1H),6.55(d, J =1.6,1H),3.92(s,2H),2.51(s,3H)
[0063] Example 3
Embodiment 3
[0065] In a 100 mL one-necked bottle, add 5-(2-fluorophenyl)-N-methyl-1-(3-pyridylsulfonyl)-1H-pyrrole-3-methylamine (6.0 g, 14.4 mmol) and acetic acid Ethyl ester (30mL) was stirred and dissolved at room temperature (15°C), methanesulfonic acid (1.2mL, 18.3mmol) was added dropwise to the reaction system, stirred at this temperature for 30 minutes, isopropanol (20mL) was added, After that, the temperature was lowered to 2°C and stirred for 30 minutes. A solid precipitated out. The solid was filtered out and washed with ethyl acetate (12 mL*2 times), and the obtained solid was dried in a vacuum oven (45°C) for 4 hours. 3.9 g of off-white solid was obtained with a yield of 55.8%.
[0066] 1 HNMR (DMSO- d 6;500MHZ)δ(ppm)8.89(d, J =4.7,1H),8.75(s,2H),8.57(s,1H),7.90(d, J =8.0,1H),7.83(s,1H),7.64(m,1H),7.53(m,1H),7.23(m,2H),7.10(m,1H),6.52(s,2H),6.39( s,4H),4.02(t,2H),2.55(t,3H),2.44(brs,6H)
[0067] Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com