Novel micafungin sodium crystal forms and preparation method thereof
A technology of micafungin sodium and crystal form, which is applied in the preparation methods of peptides, chemical instruments and methods, and cyclic peptide components, etc., can solve the problems of unsuitability for large-scale production, low solvent residues, poor fluidity, etc. , to meet the clinical drug needs, good fluidity, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of Micafungin Sodium Crystal Form I
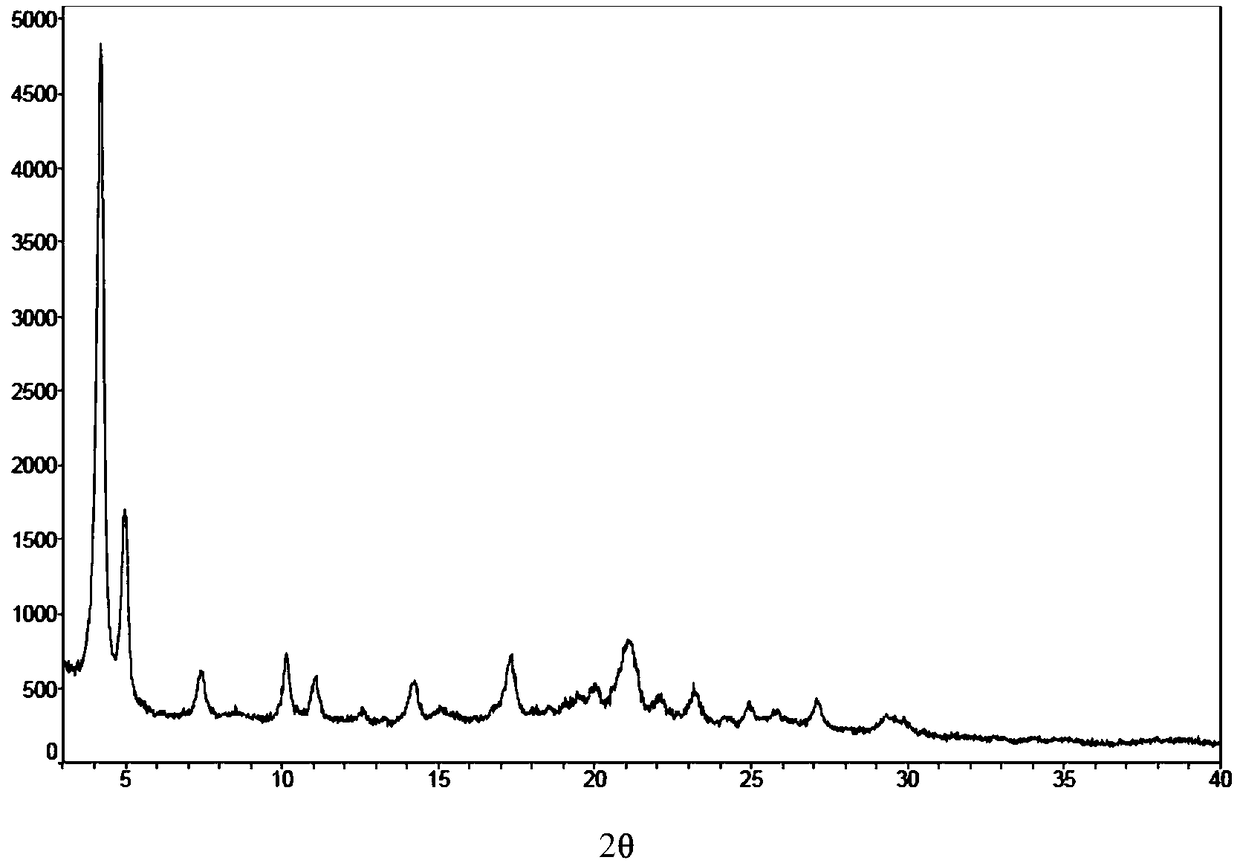
[0061] Take 10mg of amorphous micafungin sodium and dissolve it in a mixed solvent of 1mL methanol and 1mL tetrahydrofuran at 40°C, then naturally cool to 4°C, stir for 2 to 3 days, centrifuge, and dry under reduced pressure to obtain micafungin sodium crystals Type I, see the XPRD spectrum figure 1 .
Embodiment 2
[0062] Example 2 Preparation of Micafungin Sodium Crystal Form I
[0063] Take 250 mg of amorphous micafungin sodium, dissolve it in 10 mL of methanol at 50°C, add 10 mL of tetrahydrofuran, then naturally cool to room temperature, stir for 4 days, centrifuge, and dry under reduced pressure to obtain micafungin sodium crystal form I.
Embodiment 3
[0064] Example 3 Preparation of Micafungin Sodium Crystal Form I
[0065] Take 1500 mg of amorphous micafungin sodium and dissolve it in 100 mL methanol at 45° C., add 100 mL of tetrahydrofuran, and then naturally cool to room temperature, stir for 4 days, centrifuge, and dry under reduced pressure to obtain micafungin sodium crystal form I.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com