Polyamino acid grafted copolymer and preparation method thereof
A graft copolymer and polyamino acid technology, applied in the field of polyamino acids, can solve the problems of deposition, lack of stabilizer protection, etc., and achieve the effects of not easy deposition, good biocompatibility and degradability, and easy drug carrying.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The invention also provides a preparation method of the polyamino acid graft copolymer, which includes:
[0060] The polyamino acid, the hydrophilic compound, and the hydrophobic compound are reacted under the action of a condensation promoter to obtain the polyamino acid graft copolymer as shown in formula (I);
[0061] The polyamino acid is polyglutamic acid, polyaspartic acid or polyglutamic acid-polyaspartic acid copolymer;
[0062] The hydrophilic compound is any one of the compounds represented by formulas (5) to (8);
[0063] The hydrophobic compound is a polyester containing a hydroxyl group, a C8-C30 fatty alcohol or a small biological molecule with biological activity;
[0064]
[0065]
[0066] Wherein, L is independently selected from methylene or ethylene;
[0067] R 1 Is a group represented by formula (1) to formula (4);
[0068] R 2 It is a hydroxyl-containing polyester group other than one hydroxyl group, C8~C30 alkyl, aryl, C8~C30 alkene group, C8~C30 alkynyl group...
Embodiment 1~3
[0081] Add 2.63 g (0.01 mol) of γ-benzyl-L-glutamate-N-lactam acid anhydride monomer and 30 mL of anhydrous N,N-dimethylformamide into the reaction flask, and stir to dissolve. Add 0.0002mol, 0.0001mol and 0.00005mol n-hexylamine under stirring conditions, and continue the reaction for 72h under stirring at 25°C. After the reaction, the reaction mixture was settled with 300 mL of ether, filtered, and then washed three times with ether, and dried under vacuum at room temperature for 24 hours to obtain intermediate products, namely poly(γ-benzyl-L-glutamate).
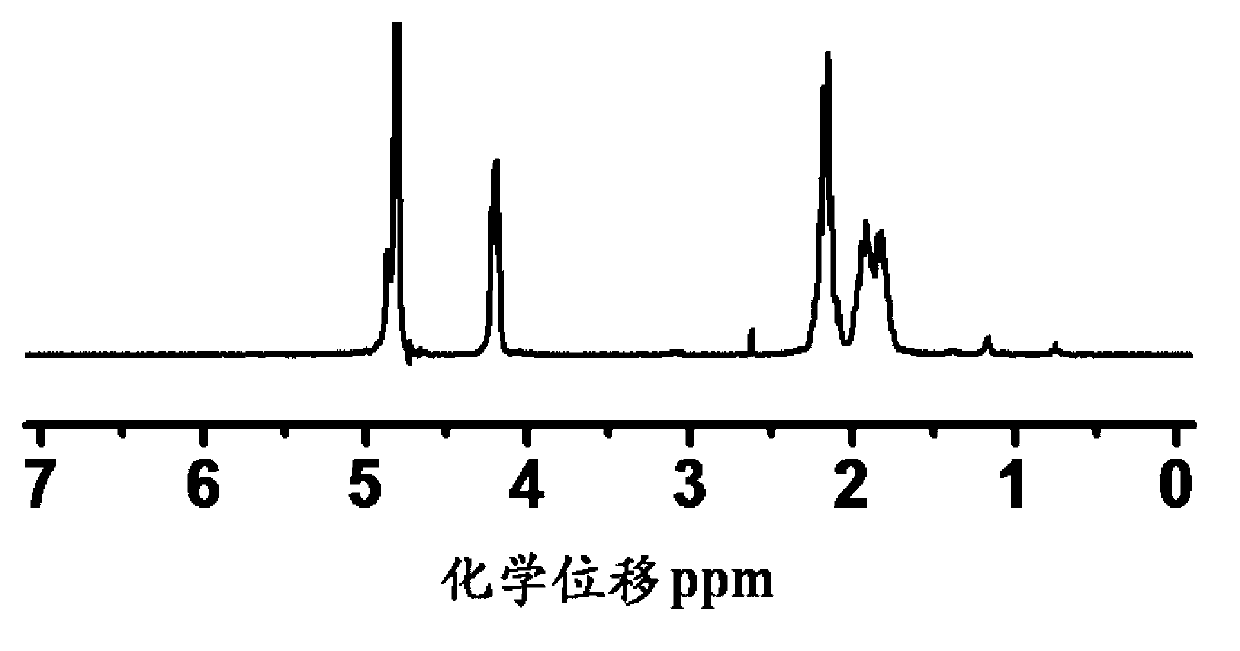
[0082] The poly(γ-benzyl-L-glutamate) was analyzed by nuclear magnetic resonance using deuterated trifluoroacetic acid as the solvent, and the number average molecular weight and average degree of polymerization were calculated according to the nuclear magnetic resonance spectrum. For the results, see Table 1.
[0083] Table 1 The number average molecular weight, average degree of polymerization and yield of the intermediate ...
Embodiment 4~6
[0091] Add 2.49g (0.01mol) of γ-benzyl-L-aspartate-N-lactam acid anhydride monomer and 30mL of anhydrous N,N-dimethylformamide into 3 reaction flasks, Stir to dissolve. Under stirring conditions, 0.0002mol, 0.0001mol and 0.00005mol n-hexylamine were added to the three reaction flasks respectively, and the reaction was continued for 72h under stirring at 25°C. After the reaction, the reaction mixture was settled with 300 mL of ether, filtered, washed with ether three times, and dried under vacuum at room temperature for 24 hours to obtain intermediate products, namely, poly(γ-benzyl-L-aspartate).
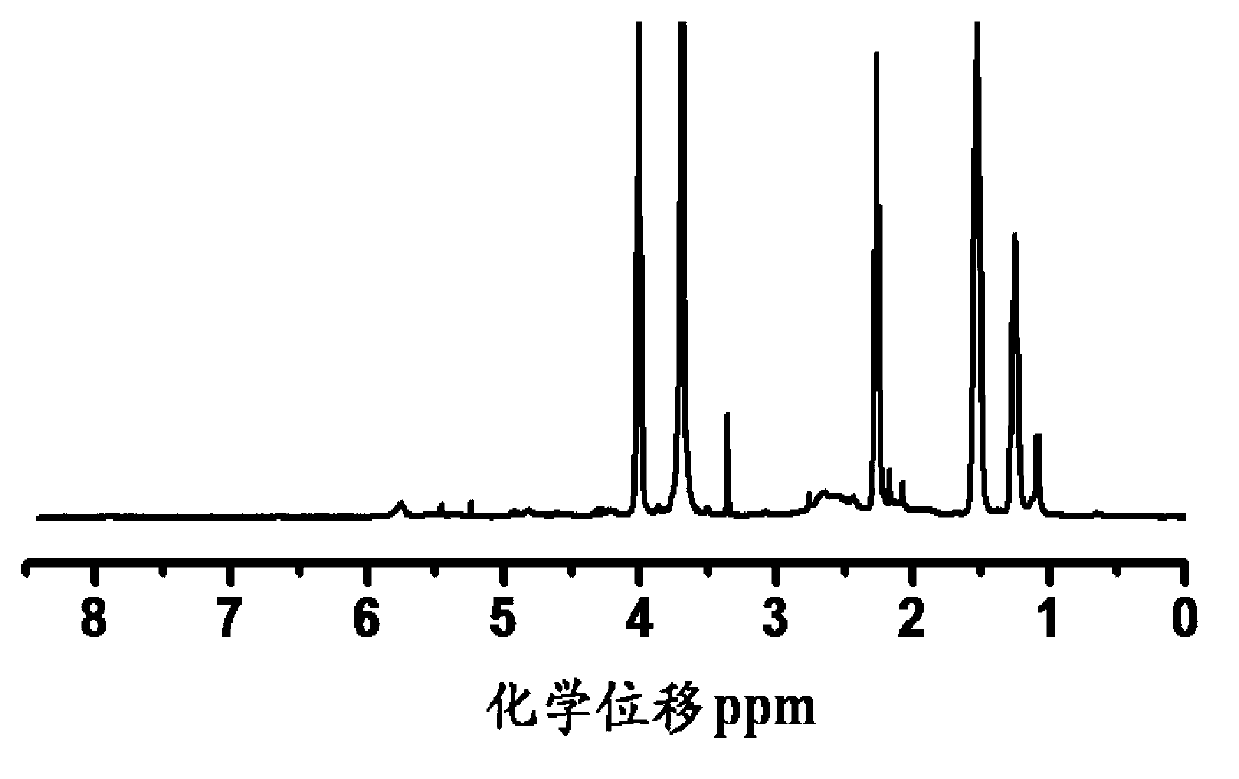
[0092] Dissolve 1g of the above-mentioned poly(γ-benzyl-L-aspartate) with 10mL of dichloroacetic acid, and add 3mL of hydrogen bromide / glacial acetic acid solution with a mass content of 33% under stirring to obtain a reaction mixture After stirring the reaction mixture at 25° C. for 1 h, the product obtained was settled with 150 mL of ether, filtered, dialyzed with water once, and lyo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com