Water-soluble organic acid salt of tegaserod as well as preparation method and application of water-soluble organic acid salt
A technology of tegaserod and organic acid salts, which is applied in the fields of organic chemistry methods, preparation of organic compounds, preparation of carbamate derivatives, etc., can solve problems such as difficulty in swallowing, and achieve the effect of meeting different clinical drug needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: 3-(5-methoxy-1H-indole-3-methylene)-N-pentyl-iminoguanidine D-pyroglutamate (2a)
[0052] Add 3-(5-methoxy-1H-indole-3-methylene)-N-pentyl-iminoguanidine (1.0g, 3.3mmol) in a 100mL single-necked bottle, add 15mL methanol, stir at room temperature After completely dissolving, D-pyroglutamic acid (0.43 g, 3.3 mmol) was added and stirred at room temperature for 1 hour. The mixture was concentrated under reduced pressure and dried to obtain crude product. Add 10 mL of ethyl acetate, stir at room temperature for half an hour, filter, collect the filter cake, and dry in a vacuum oven to obtain 1.3 g of the product.
[0053] Refining: Add 1.3g of the product to a 100mL single-necked bottle, add 20mL of diethyl ether at room temperature, stir for 0.5 hours and filter, collect the filter cake, and dry it in a vacuum oven overnight to obtain product 2a (1.2g, yield 84%). 1 H NMR (400MHz, DMSO-d 6 )δ11.47(s, 1H), 8.28(s, 1H), 7.75(s, 1H), 7.63(d, J=2.5Hz, 1H), 7.56(s...
Embodiment 2
[0054] Example 2: 3-(5-Methoxy-1H-indole-3-methylene)-N-pentyl-iminoguanidine 4-(fluorenylmethoxycarbonylamino)butyrate (2b)
[0055] Add 3-(5-methoxyl-1H-indole-3-methylene)-N-pentyl-iminoguanidine (100.0mg, 0.33mmol) into a 25mL single-necked bottle, add 5mL methanol, and stir at room temperature After it was completely dissolved, 4-(fluorenylmethoxycarbonylamino)butanoic acid (108.0 mg, 0.33 mmol) was added and stirred at room temperature for 1 hour. The mixture was concentrated under reduced pressure and dried to obtain crude product. Add 10 mL of petroleum ether, stir at room temperature for half an hour, filter, collect the filter cake, and dry in a vacuum oven to obtain 69 mg of the product.
[0056] Refining: Add 69 mg of the product to a 10 mL single-necked bottle, add 5 mL of ether at room temperature, stir for 0.5 hours and filter, collect the filter cake, and dry it in a vacuum oven overnight to obtain product 2b (52 mg, yield 25%). 1 H NMR (400MHz, DMSO-d 6 )δ1...
Embodiment 3
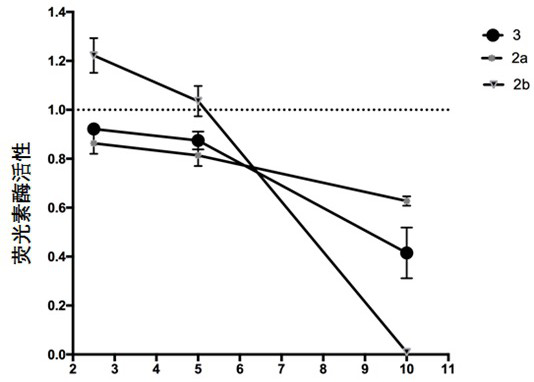
[0057] Embodiment 3: the water solubility determination of target compound
[0058] The excess compound to be tested was dissolved in water, oscillated at 37°C with a constant temperature oscillator to balance, centrifuged and filtered, and undissolved compounds were removed to obtain a saturated aqueous solution of the compound to be tested. Agilent 1260 high performance liquid chromatography was used to measure the peak area of the saturated solution, and the concentration of the compound to be tested was calculated by establishing a standard curve between the concentration of the compound to be tested and the peak area.
[0059] Liquid phase detection conditions are: Agilent TC-C18 (2) column (specification 5 μm, 250x4.6mm) detection wavelength: 278nm; mobile phase: acetonitrile / water (containing 0.05% trifluoroacetic acid)=49 / 51; injection volume The column temperature is 25°C, the flow rate is 1mL / min, and the detection time is 15min.
[0060] Table 1. Solubility of so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com