Tetrandrine liquid crystal nanoparticle preparation for eyes and preparation method thereof
A technology for tetrandrine and ophthalmic preparations, applied in the field of medicine, can solve the problems of difficulty in achieving long-term effect, large loss of drug dose, low bioavailability, etc., and achieves improved compliance, high long-term stability, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
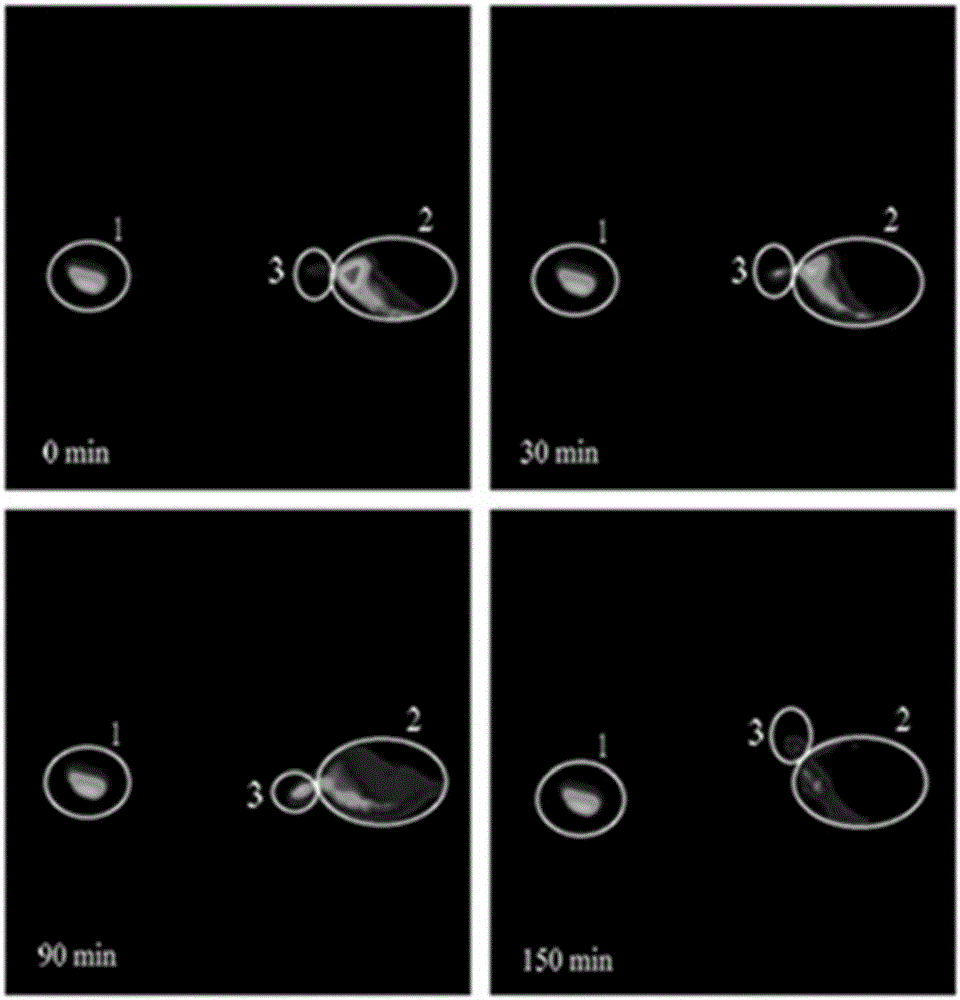
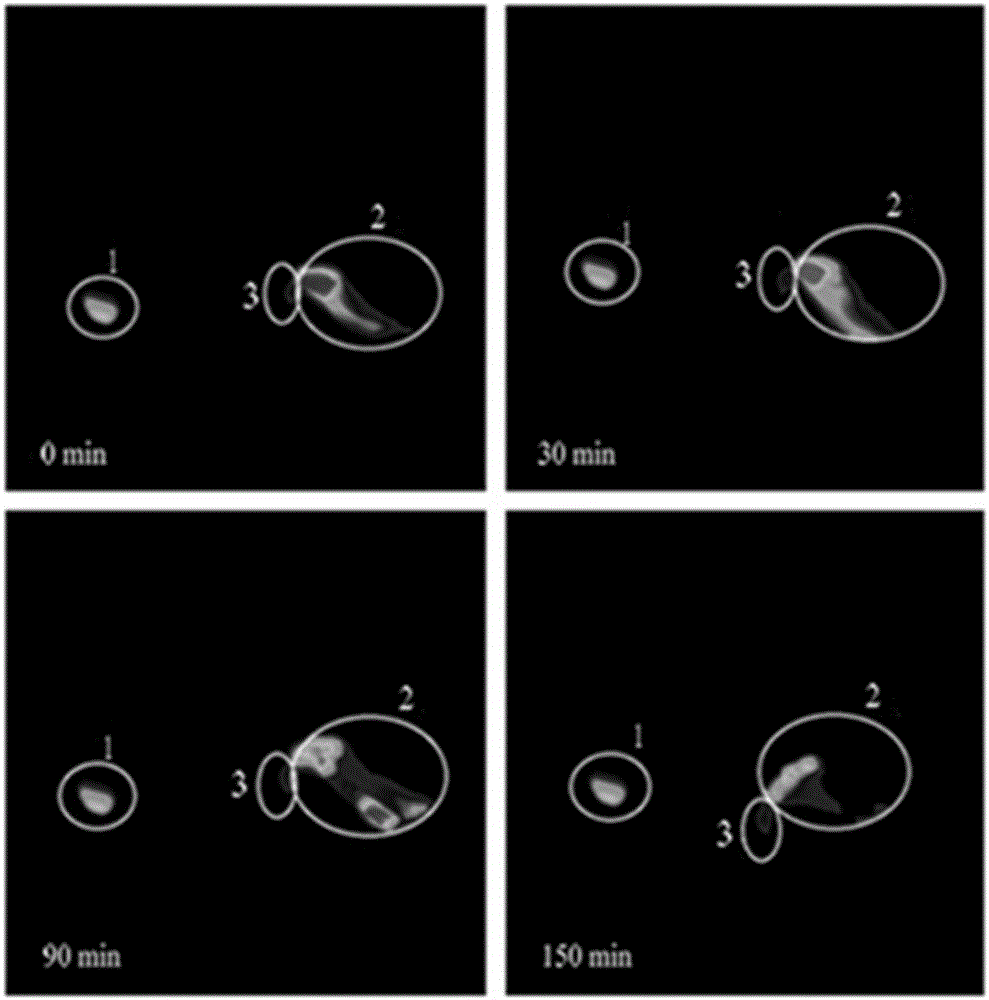
Examples
Embodiment 1
[0032] A preparation method for tetrandrine liquid crystal nanoparticle ophthalmic preparation, comprising the steps of:
[0033] (1) Take 30.8mg glycerol monooleate, 3.4mg Pluronic F127, 1mg tetrandrine, add 1mL absolute ethanol, heat to 75°C, dissolve under stirring, rotary steam under reduced pressure, remove all ethanol, and obtain a uniform oil phase;
[0034] (2) Add Gelucire44 / 14 and 3mg octadecyl carboxymethyl chitosan quaternary ammonium salt to 3mL (3g) of distilled water and stir until completely dissolved to obtain an aqueous phase; the addition amount of Gelucire44 / 14 is glycerol monooleate , Pluronic F127, tetrandrine, octadecyl carboxymethyl chitosan quaternary ammonium salt and 0.2% of the total weight of distilled water;
[0035] (3) Add the water phase to the oil phase, at 10000r·min -1 Shear and disperse for 3 minutes, cool to room temperature to obtain colostrum;
[0036] (4) Ultrasound the colostrum, the ultrasonic power is 200w, the ultrasonic wave is ...
Embodiment 2
[0039] A preparation method for tetrandrine liquid crystal nanoparticle ophthalmic preparation, comprising the steps of:
[0040] (1) Take 15mg glycerol monolinoleate, 15mg diglycerol oleate, 3.3mg pluronic F127, 1mg tetrandrine, add 0.67mL absolute ethanol, heat to 75°C, dissolve under stirring, spin under reduced pressure Steam to remove ethanol to obtain a homogeneous oil phase;
[0041] (2) Add Labrasol and 3mg carboxymethyl chitosan into 3mL (3g) distilled water and stir until completely dissolved to obtain the water phase; F127, 0.1% of the total weight of tetrandrine, carboxymethyl chitosan and distilled water;
[0042] (3) Add the water phase to the oil phase, at 5000r·min -1 Shear and disperse for 3 minutes, cool to room temperature to obtain colostrum;
[0043] (4) Ultrasound the colostrum, the ultrasonic power is 200w, the ultrasonic wave is 5s, the interval is 5s, and the ultrasonic wave is 10min in total, and the tetrandrine liquid crystal nanoparticle ophthalm...
Embodiment 3
[0045] A preparation method for tetrandrine liquid crystal nanoparticle ophthalmic preparation, comprising the steps of:
[0046] (1) Take 10mg of phosphatidylethanolamine, 20mg of dilinoleoylphosphatidylethanolamine, 12mg of 1-palmitoyl-2-oleoylphosphatidylcholine, 5mg of pluronic F127, 1mg of tetrandrine, add 1.66mL of absolute ethanol, heat to 60°C, dissolved under stirring, and rotary steamed under reduced pressure to remove all ethanol to obtain a homogeneous oil phase;
[0047] (2) Add SolutolHS15 and 5mg carboxymethyl chitosan into 5mL (5g) of distilled water and stir until completely dissolved to obtain the aqueous phase; the amount of SolutolHS15 added is phosphatidylethanolamine, dilinoleoylphosphatidylethanolamine, - 0.05% of the total weight of 2-oleoylphosphatidylcholine, Pluronic F127, tetrandrine, carboxymethyl chitosan and distilled water;
[0048] (3) Add the water phase to the oil phase, at 8000r·min -1 Shear and disperse for 3 minutes, cool to room tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
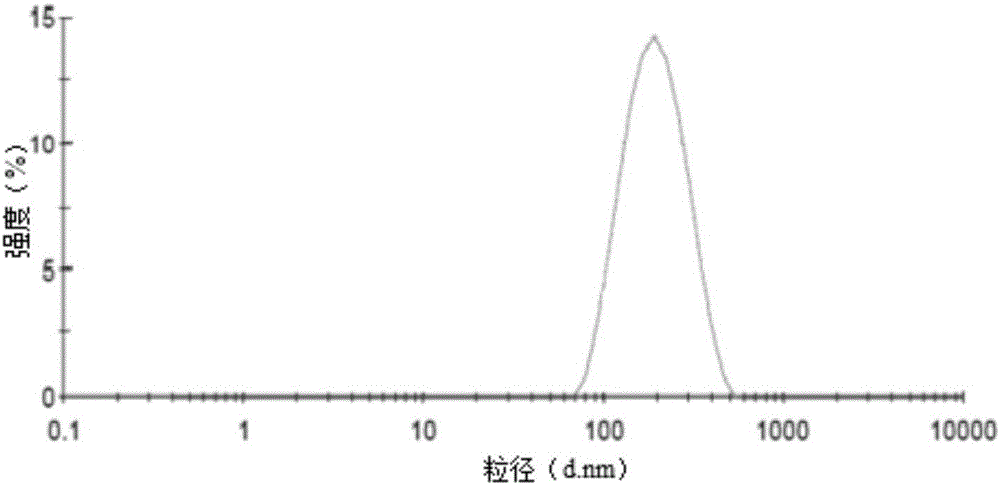
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com