Catalyst for propylene production using propane dehydrogenation and preparation method and application of catalyst
A propane dehydrogenation and catalyst technology, applied in the field of catalyst and its preparation, supported catalyst and its preparation, can solve problems such as uneven distribution, reduce catalyst acidity, increase anti-coking ability, etc., to improve catalytic efficiency, reduce transmission Mass resistance, good high temperature stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Composite carrier preparation:
[0034] Weigh 58g of P123 and dissolve in 1000ml of absolute ethanol to prepare solution 1; weigh 3.3g of zirconium nitrate and 81.6g of aluminum isopropoxide and dissolve in 500ml of 70% nitric acid aqueous solution to prepare solution 2; dissolve solution 2 within 30min Added dropwise to solution 1, stirred for 4h until completely mixed. The solution was slowly heated to 60° C., kept at the temperature for 48 hours, and the solvent was evaporated to obtain a white powder, which was calcined at 650° C. for 4 hours to obtain 21.6 g of a composite oxide carrier.
[0035] Catalyst load:
[0036] Dissolve 6.2g of chromium acetate and 0.16g of sodium nitrate in 10ml of water, add 21.6g of the above-mentioned alumina-zirconia composite oxide, stir for 30min, dry at room temperature for 18h, dry at 100°C for 12h, calcinate at 750°C for 6h, and use a tablet press Press into a sheet to obtain a propane dehydrogenation catalyst A. In the cataly...
Embodiment 2
[0038] Composite carrier preparation:
[0039] Weigh 58g of P123 and dissolve in 1000ml of absolute ethanol to prepare solution 1; weigh 4.6g of cerium nitrate and 81.6g of aluminum isopropoxide and dissolve in 500ml of 70% nitric acid aqueous solution to obtain solution 2; dissolve solution 2 within 30min Added dropwise to solution 1, stirred for 4h until completely mixed. Slowly heat the solution to 60°C, keep the temperature and heat for 42h, evaporate the solvent to obtain a white powder, and calcinate at 650°C for 4h to obtain an alumina-ceria composite oxide carrier.
[0040] Catalyst load:
[0041] Dissolve 7.4g of chromium acetate and 0.16g of sodium nitrate in 10ml of water, add 22.4g of the above-mentioned alumina-cerium oxide composite oxide, stir for 30min, dry at room temperature for 24h, dry at 100°C for 12h, and calcinate at 750°C for 6h. Press into a sheet to obtain propane dehydrogenation catalyst B. In the catalyst, the content of cerium oxide is 9.8%, the...
Embodiment 3
[0043] The preparation of the composite carrier is the same as in Example 1.
[0044] Catalyst load:
[0045] Dissolve 6.2g of chromium acetate and 0.16g of sodium nitrate in 8.2ml of water, add 21.6g of the above-mentioned alumina-zirconia composite oxide, stir for 30min, extrude, dry at room temperature for 24h, dry at 100°C for 12h, and calcine at 750°C for 6h , to prepare propane dehydrogenation catalyst C. In the catalyst, the content of zirconium oxide is 5.9%, the loading amount of chromium oxide is 10%, and the loading amount of sodium oxide is 0.3%, all of which are calculated by the weight of aluminum oxide.
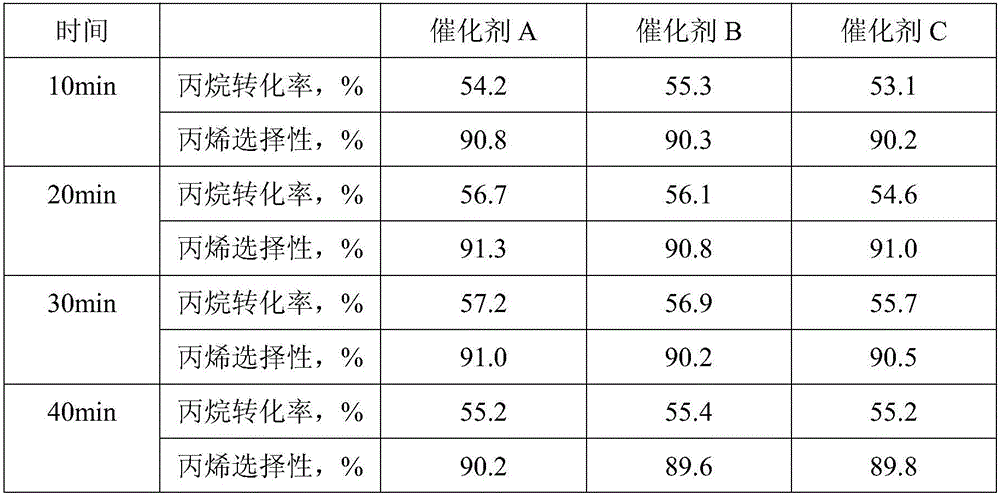
[0046] The physical properties of the catalysts obtained in the above examples are shown in Table 1.
[0047] Each embodiment of table 1 obtains the physical property of catalyst
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com