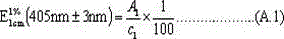Chlorins e6 metal salt compound and preparing method and application thereof
A technology of chlorin and compound, which is applied in the field of chlorin e6 metal salt compound and its preparation, can solve the problems of complex composition, limited application, poor stability, etc., and achieve the effect of clear structure and remarkable activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of chlorin e6 trisodium salt
[0030]
[0031] (1) Dissolve 100g of crude silkworm excrement chlorophyll in 300ml of ether, add 600ml of hydrochloric acid, stir for 2 hours, let stand, remove the lower layer of acid solution, add 500ml of water to dilute, add dropwise 10mol / L sodium hydroxide solution to pH 5-6, A dark green precipitate was precipitated and dried under reduced pressure to obtain about 20 g of pheophorbide a.
[0032]
[0033] (2) Take about 5g of pheophorbide a, dissolve it in 200ml of methanol, add 500ml of 0.5% sodium hydroxide methanol solution, stir for 5h, add 3g of sodium hydroxide and 30ml of water, reflux in the water bath for 2h, add hydrochloric acid dropwise To pH 5-6, add three times the volume of water, precipitate out after standing still, filter under reduced pressure and go through silica gel column chromatography (300-400 mesh, eluent: dichloromethane, methanol, acetone, formic acid = 10:1 :1:0.7), colle...
Embodiment 2
[0036] Embodiment 2: Preparation of chlorin e6 tripotassium salt
[0037]
[0038] The sodium hydroxide used in step (3) in Example 1 was replaced by potassium hydroxide to obtain chlorin e6 tripotassium salt, MS (ESI+) M / Z: 599(M+2).598( M+1), 1H-NMR (δPPM, CDCL3): 1.63(3H), 1.75(3H), 2.25-2.30(4H), 3.29(3H), 3.47(3H), 3.62(3H), 3.73-3.77(2H ), 3.80(3H), 4.56(2H), 5.01(2H), 6.15(1H), 6.34(1H), 8.05(1H), 8.86(1H), 9.59(1H), 9.77(1H).
Embodiment 3
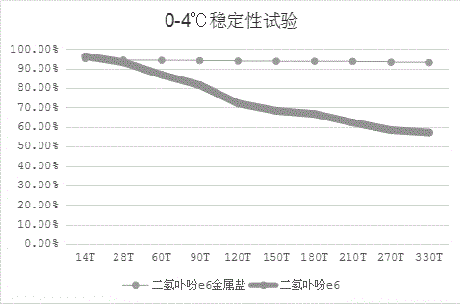
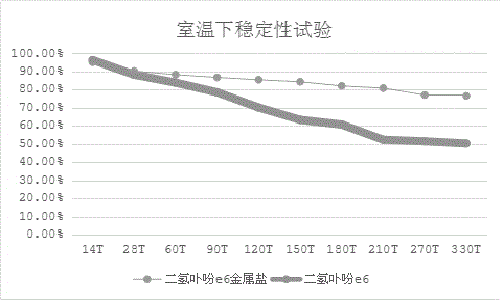
[0039] Example 3: Chlorin e6 Metal Salt Stability
[0040] Take chlorin e6 metal salt (taking sodium salt as an example) and chlorin e6 (purchased from Maya Reagent Co., Ltd.), respectively, take 1.0 g and place them in a refrigerator at room temperature and at 0-4 °C. Sampling at regular intervals measures the purity of the two by HPLC, and the results are as follows: figure 1 , figure 2 shown.
[0041] Compared with chlorin e6, the stability of chlorin e6 metal salt (taking sodium salt as an example) at room temperature and at 0-4°C is more stable, and the rate of change is slower.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com