A kind of purification method of tobramycin
A kind of technology of tobramycin and purification method, applied in the field of medicine, can solve the problem of high impurity content of tobramycin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The tobramycin activity unit in the tobramycin hydrolyzate is not less than 10000u / ml, adjust the pH to 8.0 with hydrochloric acid, calculate the amount of the column solution according to the adsorption capacity of the D151 resin chromatography column, and use 3.0- Wash with 5.0% ammonium sulfate to remove impurities, and wash according to 25% of the amount on the column. After washing, use 0.1-2.0mol / L ammonia water gradient elution, the initial flow rate of ammonia water elution is 1200-1400ml / min, every 4 Measure the flow once an hour. When the mixture of kanamycin B and tobramycin begins to appear, start to collect in sections. Change the flow of ammonia elution to 450-550ml / min. Measure the flow every 2 hours. TLC method is used for the whole process Take control.
[0028] Start the vacuum system, condensation system. When the vacuum degree is ≤-0.07MPa, concentrate while feeding, control T≤70°C, and control the volume of the concentrated solution to be between 0...
Embodiment 2
[0030] Get the concentrated solution that embodiment 1 obtains, be divided into 2 batches, carry out the test of experiment A and experiment B respectively, experimental procedure and experimental result are as follows:
[0031] Experiment A:
[0032] Ammonia water adjusts the pH value of the concentrated solution of Example 1 to 8.00, passes through the D151 resin chromatography column, washes and removes impurities with 3% ammonium sulfate solution, washes by 25% of the amount on the column, and controls the flow rate at 0.40 ± 0.1BV to Thin-layer chromatography detects that when the color spot of the washing impurity component should be ≤ 2% of the TB color spot, stop washing, elute with ammonia water, and collect the ammonia water eluate.
[0033] Experiment B:
[0034] Ammonia water adjusts the pH value of the concentrated solution of Example 1 to 8.00, passes it through the D151 resin chromatography column, washes and removes impurities with 3%-5% ammonium sulfate solut...
Embodiment 3
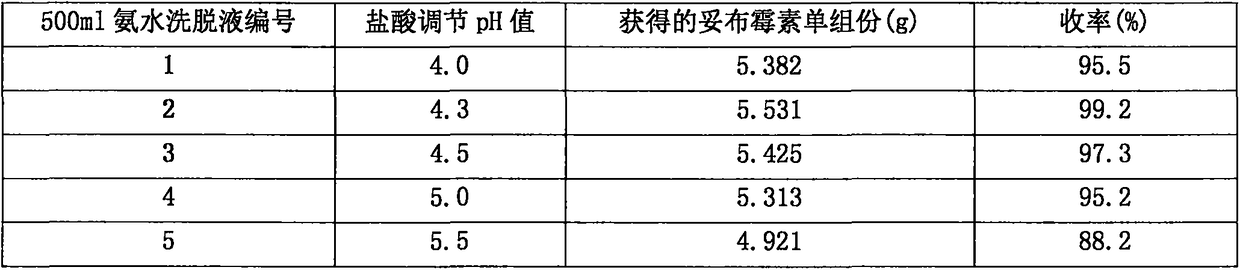
[0044] Get 6 parts of the ammonia eluent obtained in Experiment B of Example 2 and concentrate, and concentrate 500ml of the ammonia eluent obtained in Experiment B of Example 2, and adjust the pH value to 4.0, 4.3, 4.5, 5.0, 5.5, respectively, with hydrochloric acid. 6.0, into the D122 resin chromatography column, the experimental conditions of the D122 resin chromatography column are glass column diameter-to-height ratio 1:8, resin load 500ml, pure water elution flow rate 100ml / h, collect the eluate. Concentrate and spray dry to obtain tobramycin. The results are shown in Table 2.
[0045] Table 2
[0046]
[0047]
[0048] Conclusion: 5.578g of tobramycin single component in the ammonia eluent obtained in Experiment B of Example 2, and when the pH value reaches 4.0, 4.3, 4.5, 5.0, 5.5, 6.0 respectively, the obtained tobramycin single component The components are 5.382g, 5.531g, 5.425g, 5.313g, 4.921g, 4.529g, and the yields are 96.5%, 99.2%, 97.3%, 95.2%, 88.2%, 81.2%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com