Chitosan-based MRI contrast medium and preparation method
A chitosan and contrast agent technology, applied in the field of medical clinical magnetic resonance imaging, can solve the problems of short cycle time and tissue residence time, high biological toxicity, low relaxation efficiency, etc., and achieve simple preparation method and easy biodegradation , the effect of high relaxation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
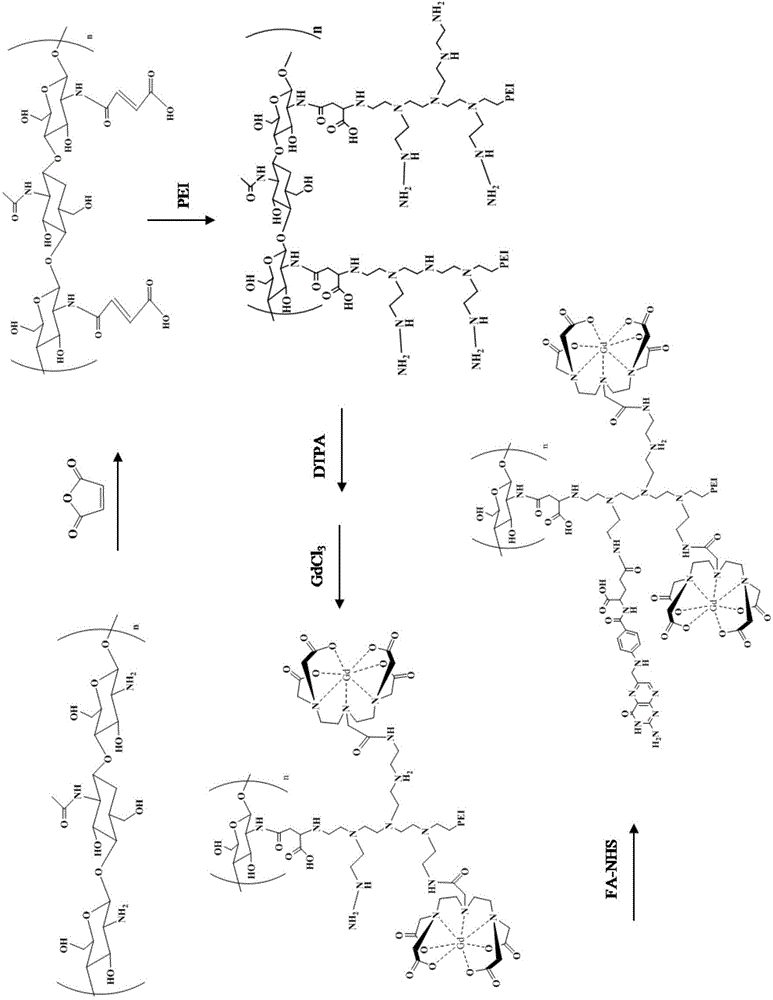
[0036] Embodiment 1 The preparation method of the targeted MRI contrast agent based on chitosan, the specific steps are as follows:
[0037] (1) Chitosan pretreatment: Chitosan was dissolved in glacial acetic acid solution with a concentration of 0.1M, and then sodium hydroxide (NaOH) with a concentration of 0.2M was added to the above solution to obtain chitosan precipitation. The precipitate was then washed several times with water until pH = 7.0.
[0038] (2) Disperse the treated chitosan in the DMSO solution, and add the DMSO solution containing maleic anhydride dropwise into the above. After the dropwise addition, the mixed solution was reacted in an oil bath at 60°C for 8h. The product was precipitated with acetone and dried under vacuum to obtain N-maleylated chitosan (NMC).
[0039] (3) Dissolve N-maleylated chitosan completely in 0.25% sodium hydroxide solution, then add polyethyleneimine-containing aqueous solution dropwise into the above solution, and react at 60°...
Embodiment 2
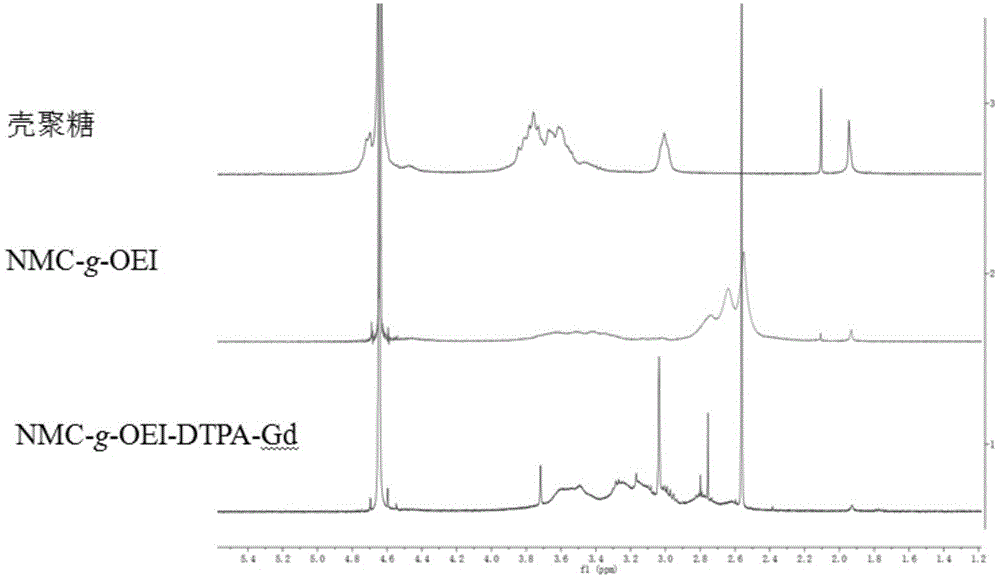
[0043] Embodiment 2 measures NMC, NMC-g-PEI and NMC-g-PEI-DTPA 1 HNMR chart, including: test NMC, NMC-g-PEI and NMC-g-PEI-DTPA respectively with heavy water as solvent 1 HNMR diagram, such as figure 2 shown.
Embodiment 3
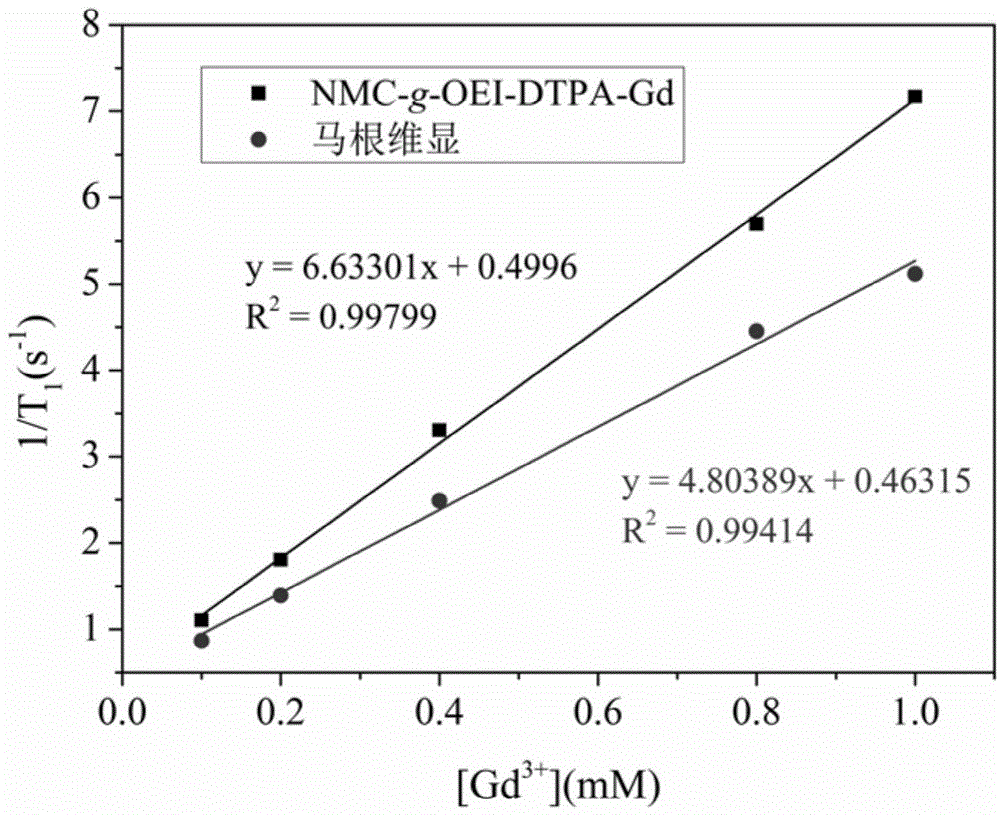
[0044] Example 3 The polymer contrast agent of the present invention and the relaxation time T of Gd-DTPA are tested on an MRI tester at 11.7T 1 and T 1 Weighted imaging, including:
[0045] Prepare above-mentioned two kinds of samples of 0.1, 0.2, 0.4, 0.8, 1.0mM respectively, after the test, take the concentration as the abscissa and the reciprocal of the relaxation time as the ordinate to carry out linear fitting to obtain the contrast agent of the present invention and Gd-DTPA Relaxation rates were 6.6mM -1 ·s -1 and 4.8mM -1 ·s -1 ( image 3 ). The relaxation rate of the contrast agent of the present invention is obviously higher than that of Gd-DPTA. Through the T of both at different concentrations 1 weighted imaging ( Figure 4 ) It can be seen that both of them tend to become brighter with the increase of the concentration, but the contrast effect of the contrast agent of the present invention is obviously brighter than that of Gd-DTPA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com