Supported catalyst used for butylene oxidative dehydrogenation to prepare butadiene, and preparation method thereof
A supported catalyst, oxidative dehydrogenation technology, applied in the field of catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
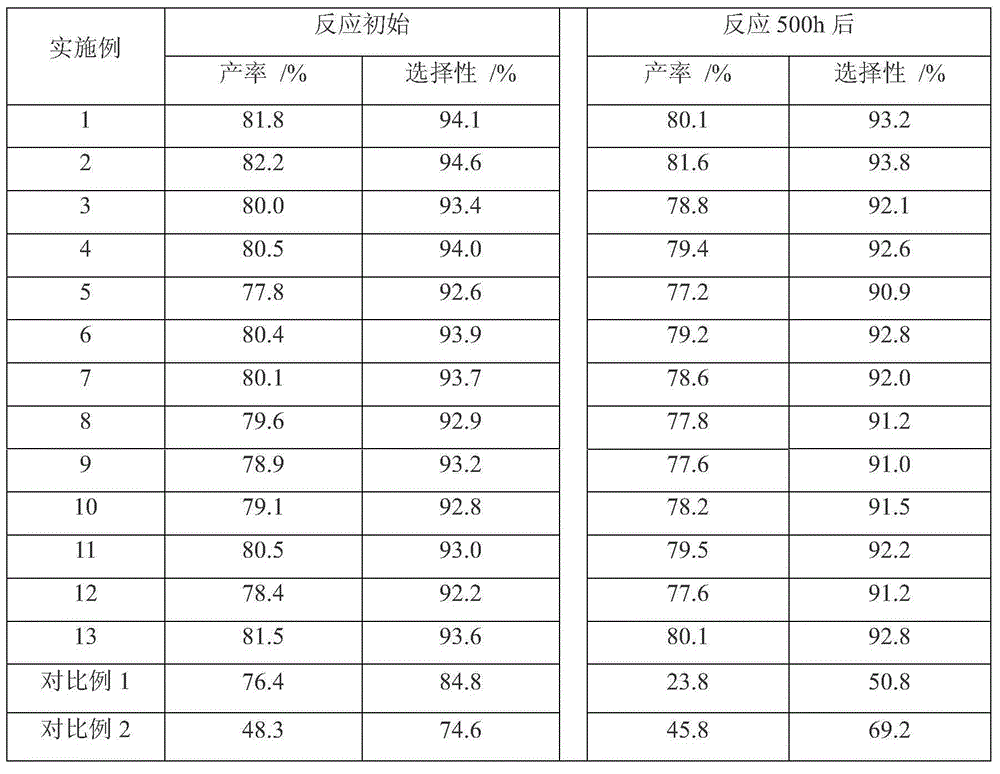
Embodiment 1
[0044] (1) First, grind the metal precursors A copper sulfate 996g, B cobalt nitrate 58.5g, C calcium chloride 66g into 40 mesh microspheres; secondly, divide the copper sulfate into 598g and 398g according to the ratio of 60% and 40% two servings. Mix cobalt nitrate and calcium chloride well.
[0045] (2) configure the ferric nitrate solution of 20L 0.4mol / L, under stirring condition, gradually add the copper sulfate of 598g into the ferric nitrate solution, react for 30 minutes, then slowly add the mixture of cobalt nitrate and calcium chloride, react 90 398g of copper sulfate was added after 10 minutes, and 90g of activated carbon and 44g of Astragalus powder were added after 40 minutes of reaction;
[0046] (3) After stirring the slurry for 30 minutes, add 15% ammonia water dropwise to the slurry to adjust the pH value of the slurry to 8.0;
[0047] (4) thermally modifying the slurry by placing the slurry at a constant temperature of 85°C for 90 minutes;
[0048] (5) fi...
Embodiment 2
[0053] The catalyst preparation process is as in Example 1, with 20L of 0.6mol / L ferric nitrate solution, the metal precursor is ground into 80-mesh microspheres, the precursor A is divided into two parts of 65% and 35%, and the metal precursor is added twice at intervals. The reaction times were respectively 90 minutes and 40 minutes, and the amounts of the metal precursors were: 1245 g of copper sulfate, 17 g of manganese sulfate, and 54 g of strontium chloride. After 60 minutes of reaction, 94 g of activated carbon and 94 g of polyacrylamide were added. After stirring for 40 minutes, 15% ammonia water was added dropwise to the slurry, the pH value of the slurry was adjusted to 9.0, and the slurry was thermally modified at a constant temperature of 85° C. for 90 minutes. The slurry was filtered and washed with tap water to bring the pH of the slurry to 7.0. The slurry was filtered, fired at 180°C for 6 hours, 220°C for 8 hours, 380°C for 3 hours, and 490°C for 2 hours. Gri...
Embodiment 3
[0055] The catalyst preparation process is as in Example 1, 10L of 1.0mol / L ferric nitrate solution is prepared, the metal precursor is ground into 60 mesh microspheres, the precursor A is divided into two parts of 70% and 30%, and the metal precursor is added twice at intervals. The reaction times were 70 minutes and 70 minutes, respectively, and the amounts of the metal precursors were: 893 g of zinc nitrate, 88 g of cobalt nitrate, and 44 g of calcium chloride. After 50 minutes of reaction, 125 g of activated carbon and 25 g of saffron powder were added. After stirring for 60 minutes, 20% ammonia water was added dropwise to the slurry, the pH value of the slurry was adjusted to 8.5, and the slurry was thermally modified at a constant temperature of 85° C. for 120 minutes. The slurry was filtered and washed with desalinated water to bring the pH of the slurry to 7.2. The slurry was filtered, fired at 150°C for 12 hours, 290°C for 4 hours, 380°C for 2 hours, and 470°C for 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com