Related substance analysis method of terazosin hydrochloride tablets
A technology of terazosin hydrochloride tablets and related substances, which is applied in the field of analysis and detection of related substances of terazosin hydrochloride tablets, can solve problems such as no reports of terazosin hydrochloride tablets, and achieve qualified inspection and peak purity , high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
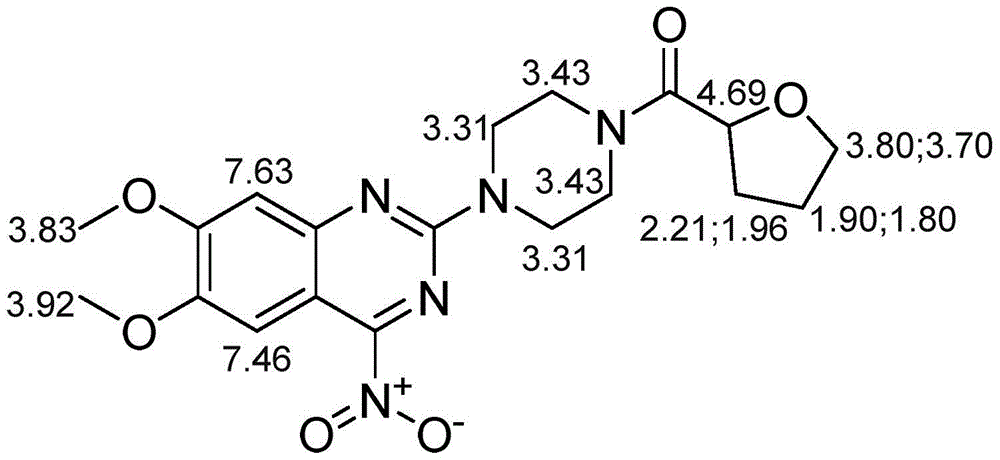
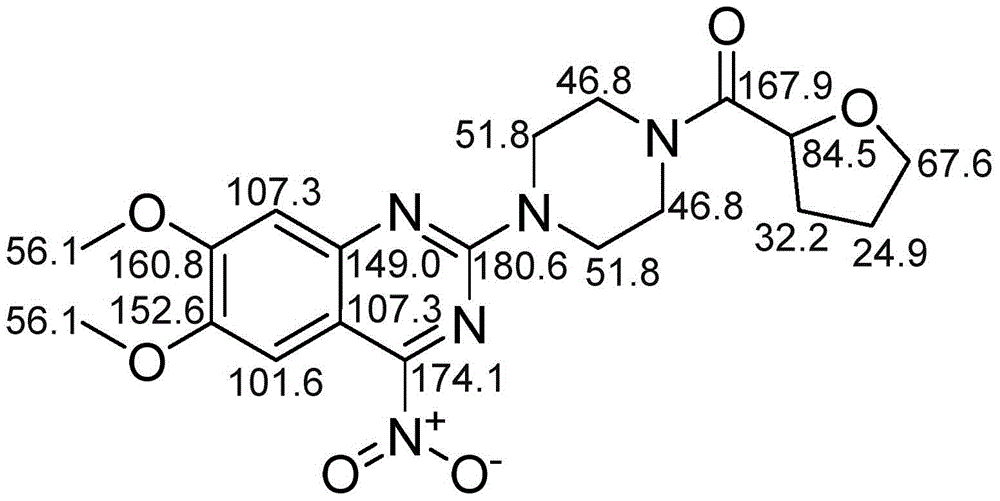
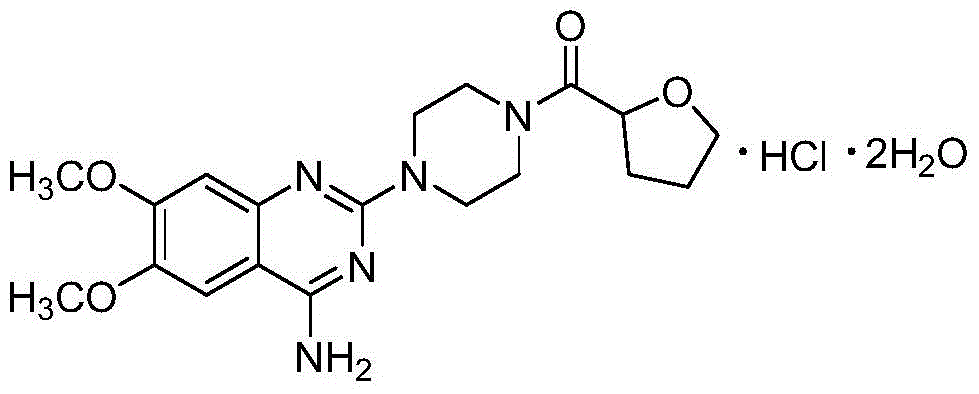
[0038] Example 1: Preparation and structural confirmation of related substances
[0039] Get 10g of terazosin hydrochloride raw material and spread evenly in the petri dish, place in oven, 85 ℃ destroy 42 hours. Then, the high-temperature destruction sample was dissolved in methanol-water solution with a methanol volume percentage concentration of 80%, stirred for 20 minutes, filtered with a suction filter funnel, collected the filtrate, and concentrated under reduced pressure to obtain 6.2 g of a concentrate; then the concentrate was used Dissolve 8ml of methanol, mix the sample with 8g of normal-phase silica gel with a particle size of 100-200 mesh, evaporate the solvent, use 120g of normal-phase silica gel with a particle size of 200-300 mesh as separation silica gel for column chromatography, and use chloroform with a volume ratio of 7:2 -Isocratic elution with methanol mixed solvent, collect the 11th column volume eluate, concentrate under reduced pressure to obtain 4.8g ...
Embodiment 2
[0043] Embodiment 2: the preparation of related substance
[0044] Get 10g of terazosin hydrochloride raw material and spread evenly in the petri dish, place in oven, 80 ℃ destroy 48 hours. Then, the high-temperature destruction sample was dissolved in methanol-water solution with a concentration of 70% by volume of methanol, stirred for 20 minutes, filtered with a suction filter funnel, collected the filtrate, and concentrated under reduced pressure to obtain 6.1 g of concentrate; Dissolve 8ml of methanol, mix the sample with 8g of normal-phase silica gel with a particle size of 100-200 mesh, evaporate the solvent, use 120g of normal-phase silica gel with a particle size of 200-300 mesh as separation silica gel for column chromatography, and use chloroform with a volume ratio of 5:1 -Isocratic elution with methanol mixed solvent, collect the 14th column volume eluate, concentrate under reduced pressure to obtain 4.5 g of crude product; then dissolve the crude product with ace...
Embodiment 3
[0045] Embodiment 3: the preparation of related substance
[0046] Get 10g of terazosin hydrochloride raw material and spread evenly in the petri dish, place in oven, 90 ℃ destroy 36 hours. Then, the high-temperature damage sample was dissolved in methanol-water solution with a methanol volume percentage concentration of 90%, stirred for 20 minutes, filtered with a suction filter funnel, collected the filtrate, and concentrated under reduced pressure to obtain 6.4 g of concentrate; Dissolve 8ml of methanol, mix the sample with 8g of normal-phase silica gel with a particle size of 100-200 mesh, evaporate the solvent, use 120g of normal-phase silica gel with a particle size of 200-300 mesh as separation silica gel for column chromatography, and use chloroform with a volume ratio of 2:1 - Isocratic elution with methanol mixed solvent, collect the 8th column volume eluate, concentrate under reduced pressure to obtain 4.9 g of crude product; then dissolve the crude product with ace...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com