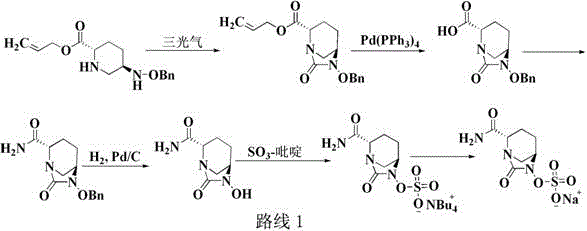
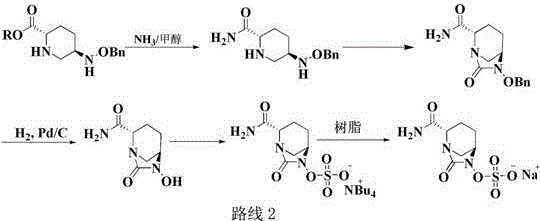
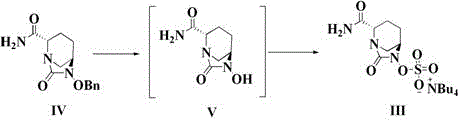
Preparation method of improved avibactam sodium intermediate compound
A compound and sulfonation technology, applied in the field of medicinal chemistry, can solve the problems of being unsuitable for industrial scale-up production, cumbersome reaction operation process, high hydrogen reduction risk, avoiding hydrogenation catalytic operation, reducing safety risks, improving product yield and The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 , ({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-octane-6 Preparation of -yl]oxy}sulfonyl)tetrabutylammonium salt (compound III)
[0034] Add isopropanol (650ml) and purified water (650ml) into the reaction flask, add compound IV (140.0g) and palladium carbon (14.0g), control the temperature at 5-15°C, add triethylsilane (139.0g) dropwise , TLC detects that the reaction is complete and then suction filtered and washed. Triethylamine (13.1 g) and trimethylamine sulfur trioxide (79.6 g) were added to the filtrate. The temperature was controlled at 5-15°C, and the reaction was stirred for 2 hours. A 45% aqueous solution of tetrabutylammonium hydroxide (235.0 g) was added. Dichloromethane was added to the reaction solution for extraction, dried over anhydrous sodium sulfate, filtered with suction, washed, and concentrated to obtain an oil. Add 700ml of methyl tert-butyl ether, stir and crystallize, filter with suction, wash, and dry t...
Embodiment 2
[0035] Example 2 , ({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-octane-6 Preparation of -yl]oxy}sulfonyl)tetrabutylammonium salt (compound III)
[0036] Add ethanol (650ml) and purified water (650ml) into the reaction flask, add compound IV (140.0g) and palladium carbon (14.0g), control the temperature at 5-15°C, add dropwise triethylsilane (139.0g), TLC After the detection reaction is complete, filter with suction and wash. Triethylamine (13.1 g) and trimethylamine sulfur trioxide (79.6 g) were added to the filtrate. The temperature was controlled at 5-15°C, and the reaction was stirred for 2 hours. A 45% aqueous solution of tetrabutylammonium acetate (235.0 g) was added. Dichloromethane was added to the reaction solution for extraction, dried over anhydrous sodium sulfate, filtered with suction, washed, and concentrated to obtain an oil. Add 700ml of methyl tert-butyl ether, stir and crystallize, filter with suction, wash, and dry to obtain 2...
Embodiment 3
[0037] Example 3 , ({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-octane-6 Preparation of -yl]oxy}sulfonyl)tetrabutylammonium salt (compound III)
[0038] Add isopropanol (650ml) and purified water (650ml) into the reaction flask, add compound IV (140.0g) and palladium carbon (14.0g), control the temperature at 5-15°C, add triethylsilane (139.0g) dropwise , TLC detects that the reaction is complete and then suction filtered and washed. Triethylamine (13.1 g) and trimethylamine sulfur trioxide (79.6 g) were added to the filtrate. The temperature was controlled at 5-15°C, and the reaction was stirred for 2 hours. A 45% aqueous solution of tetrabutylammonium acetate (235.0 g) was added. Dichloromethane was added to the reaction solution for extraction, dried over anhydrous sodium sulfate, filtered with suction, washed, and concentrated to obtain an oil. Add 700ml of methyl tert-butyl ether, stir and crystallize, filter with suction, wash, and dry to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com