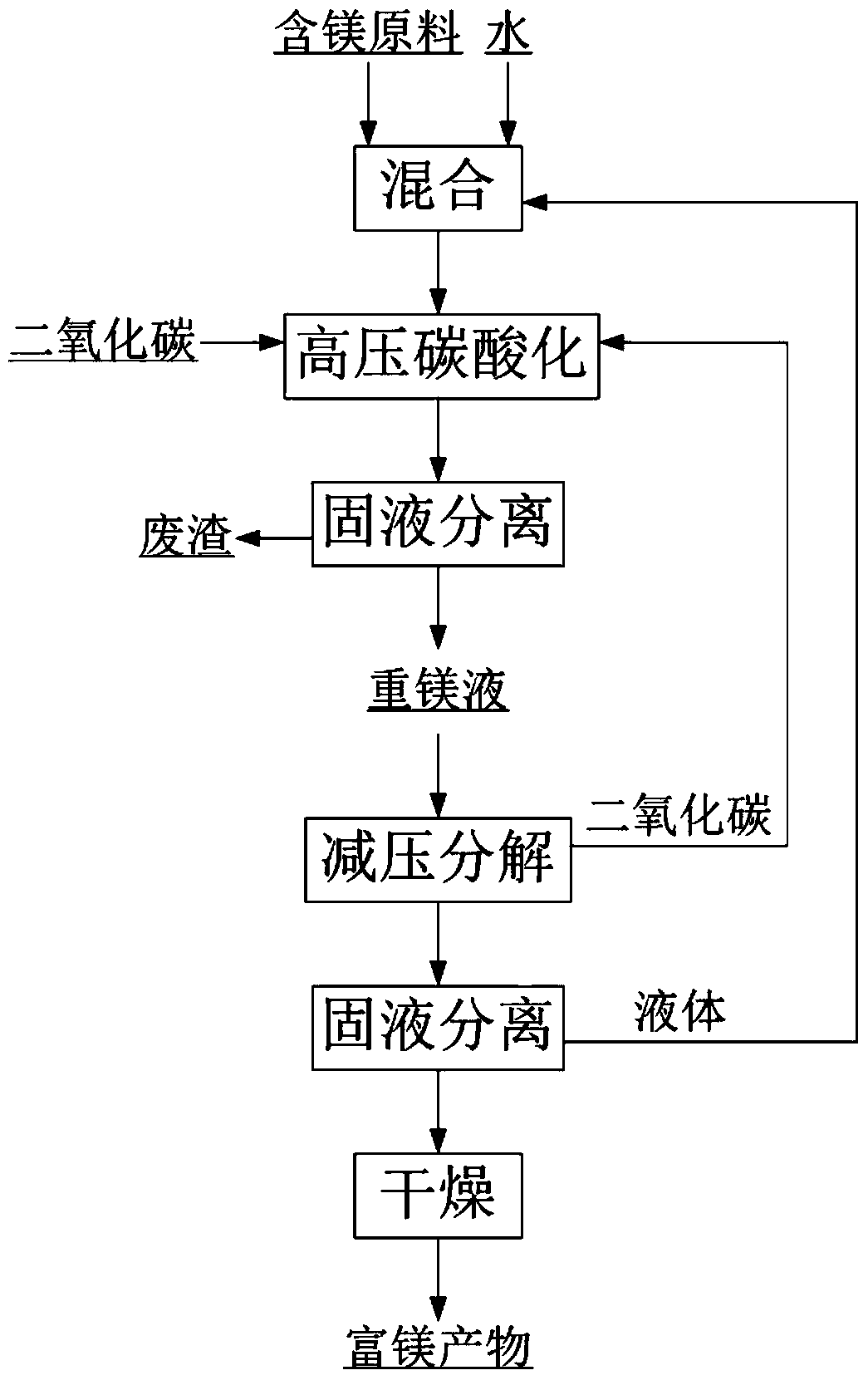
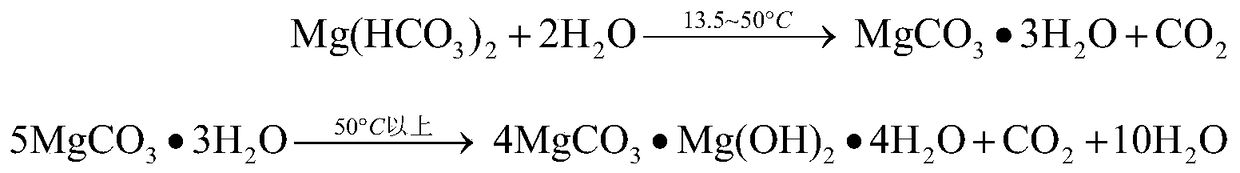A kind of method for extracting magnesium by high-pressure carbonization
A high-pressure, magnesium oxide technology, applied in the field of high-pressure carbonization to extract magnesium, can solve the problems of high energy consumption in pyrolysis and low concentration of heavy magnesium solution, and achieve the effects of small equipment size, simple process steps, and resource saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The composition is MgO 38.13%, SiO 2 13.80%, CaO 1.30%, B 2 o 3 0.82%, TFe 6.87%, Al 2 o 3 13.5g of 1.77% soda boron mud (wherein the magnesium exists in forms such as magnesium hydroxide, serpentine) is mixed with 75g water to form a reaction slurry (magnesium that can be carbonized and extracted in the raw material (in terms of magnesium hydroxide) The mass ratio of water to water is 1:20), carbon dioxide is introduced, and the pressure is 5MPa, the stirring speed is 500rpm, and the temperature is 70°C. The reaction is 1.5h, and the heavy magnesium solution is obtained by filtering under the pressure of 5MPa. The concentration of the heavy magnesium solution (calculated as MgO) is 16.571g / L, impurity calcium ion concentration is 0.130g / L, magnesium extraction rate is 24.1%, the heavy magnesium solution is slowly reduced to normal pressure at 70°C and stirred for 1.5h, filtered and dried at 80°C After 48 hours, 2.6 g of basic magnesium carbonate with a magnesium ox...
Embodiment 2
[0047] The composition is MgO 85.85%, SiO 2 5.54%, CaO 1.76%, TFe 0.85%, Al 2 o 3 3.5g of 1.60% magnesite lightly burned powder (wherein the magnesium exists in forms such as magnesium oxide) is mixed with 75g water to form a reaction slurry (the amount of magnesium (calculated as magnesium hydroxide) that can be extracted by carbonization in the raw material and water The mass ratio is 1:17), passing carbon dioxide, reacting for 1.5h at a pressure of 5MPa, a stirring speed of 500rpm, and a temperature of 30°C, and filtering under a pressure of 5MPa to obtain a concentration of heavy magnesium solution (calculated as MgO) of 32.96g / L , the impurity calcium ion concentration is 0.18g / L, and the magnesium extraction rate is 82.3%. The heavy magnesium solution is quickly reduced to normal pressure at 30°C and left for 0.5h. After filtering, it is dried at -58°C for 8h to obtain magnesium oxide with a magnesium oxide content of 28.79%. Magnesium carbonate trihydrate 7.8g.
Embodiment 3
[0049] The composition is MgO 36.98%, SiO 2 4.77%, CaO 57.97%, TFe 1.25%, Al 2 o 3 8.1g of 1.02% dolomite lightly burned powder (magnesium therein exists in forms such as magnesium oxide) is mixed with 75g water to form a reaction slurry (the quality of magnesium (calculated as magnesium hydroxide) and water that can be extracted by carbonization in the raw material Ratio of 1:17), passing carbon dioxide, reacting at a pressure of 5MPa, a stirring speed of 500rpm, and a temperature of 50°C for 1.5h, and filtering under a pressure of 5MPa to obtain a concentration of heavy magnesium solution (calculated as MgO) of 18.04g / L, The impurity calcium ion concentration is 0.07g / L, and the magnesium extraction rate is 45.2%. The heavy magnesium solution is rapidly reduced to 1MPa at 25°C and stirred for 1h, filtered and dried at -70°C for 8h to obtain magnesium carbonate trihydrate with a magnesium oxide content of 28.67%. 3.0g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com