Building method for mouse model systemically infected with pseudomonas aeruginosa
A Pseudomonas aeruginosa and mouse model technology, applied in the biological field, can solve the problems of not being able to represent the infection situation, and achieve the effects of easy control of infection conditions, high infection rate, and low bacterial concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: preparation of Pseudomonas aeruginosa bacterium liquid
[0034] Take the frozen Pseudomonas aeruginosa clinical strain XN-1, revive it with LB nutrient agar plate, and culture it aerobically at 37°C overnight. Pick a single colony and inoculate 20ml of LB liquid medium, and incubate with aerobic shaking at 180rpm at 37°C for 15h, then inoculate 0.2mL into 20ml of LB liquid medium, and inoculate at 37°C with aerobic shaking at 230rpm for 6h. The cells were collected by centrifugation at 6000g, washed twice with normal saline, and then resuspended with normal saline. Adjust the bacterial concentration to OD 600 The value is 0.8, and the bacterial concentration measured by plate counting method is 3.0×10 9 CFU / ml.
Embodiment 2
[0035] Example 2: Establishment of mouse tail vein injection scheme
[0036] In the infection experiment of the whole body model of Pseudomonas aeruginosa, the control of the intravenous dose is the guarantee of the stability of the model. In order to better control the intravenous dose and prevent the bacterial liquid from entering the soft tissue around the blood vessel, the following measures have been taken: (1 ) Irradiating the mouse with infrared light for 3 minutes, it can be seen that the veins on the left and right sides of the tail of the mouse are obviously filled and expanded; (2) Fix the mouse with a mouse fixer, and insert the needle at a distance of 2 to 3 cm from the tail of the mouse at less than 30 degrees; (3) Disposable sterile insulin syringes are used, with short and thin needles; (4) There will be a sense of void when the needle enters the blood vessel, and there is no resistance when pushing, and blood will return when the needle is pulled out; (5) In th...
Embodiment 3
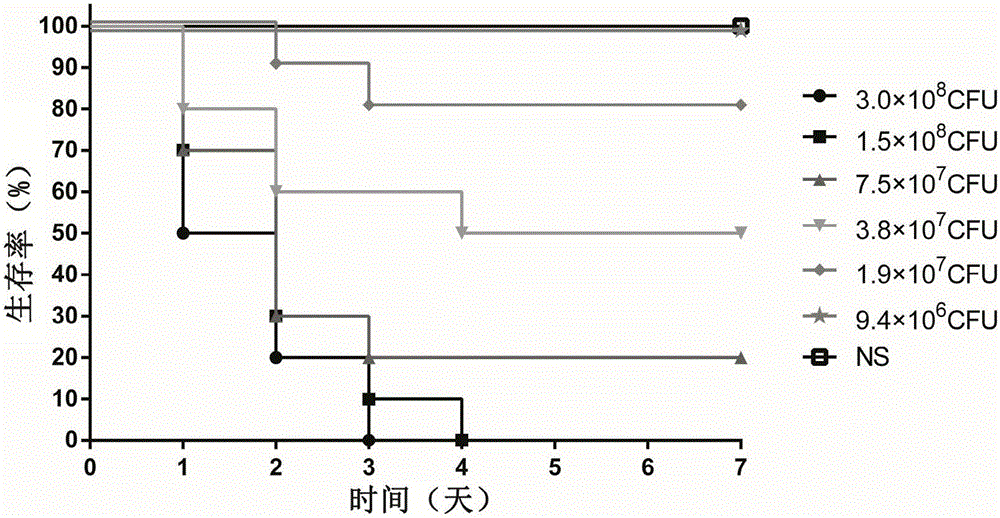
[0037] Example 3: Determination of Infection Dose
[0038] Physiological saline was used to adjust the PAXN-1 bacterial solution obtained in Example 1 to six different concentrations, and the experimental animal female BALB / c mice were randomly divided into 7 groups, and were infected by tail vein injection. The infection dose of each mouse was 100 μL, and the same dose of normal saline (NS) was used as a blank control. The grouping and infection status of the animals are shown in Table 1. After the infection, the death of the mice was observed every other day, and the observation period was 7 days. After the observation period, the remaining animals were treated with CO 2 Euthanized by inhalation.
[0039] Table 1: Determination of Infection Dose in Pseudomonas aeruginosa Systemic Model
[0040]
[0041]
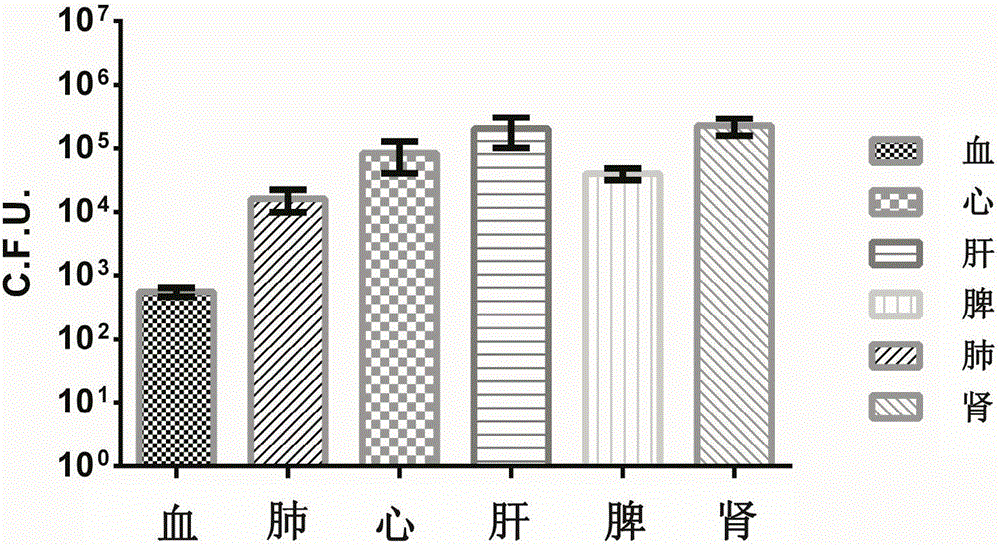
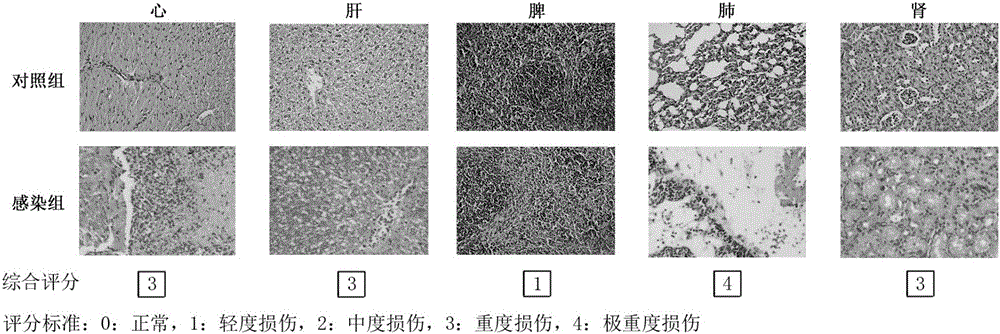
[0042] When adopting the Pseudomonas aeruginosa bacterial strain PAXN-1 infection BALB / c mouse of different concentrations (2-fold gradient dilution), through 7 da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com