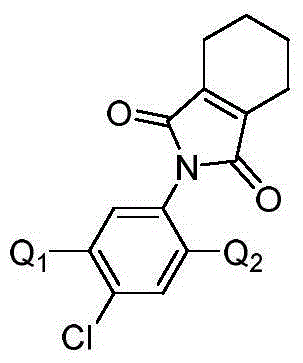
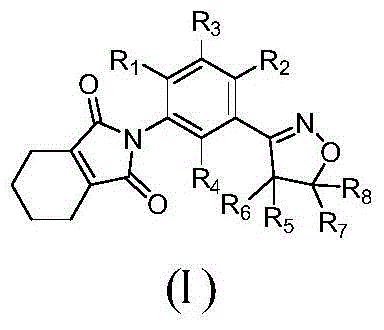
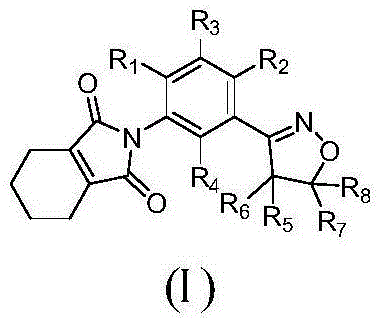
Isoxazoline-containing tetrahydrophthalimide compound and use thereof
A kind of technology of tetrahydrophthalimide and isoxazoline, applied in the field of tetrahydrophthalimide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] The preparation of embodiment 1 compound 6
[0125]
[0126] 1) Preparation of 2-chloro-4-fluoro-5-nitrobenzaldehyde oxime
[0127] Dissolve 42g (0.206mol) of 2-chloro-4-fluoro-5-nitrobenzaldehyde in 200ml of ethanol, drop to 0°C, add dropwise 17.4g (0.25mol) of an aqueous solution of hydroxylamine hydrochloride under stirring, and then raise to room temperature Stir the reaction. After 2 hours, TLC monitored the reaction to be complete. Poured into water, filtered to obtain 38.3g of white solid. The melting point is 103°C.
[0128] 2) Preparation of ethyl 3-(2-chloro-4-fluoro-5-nitrophenyl)-5-methyl-4,5-dihydroisoxazole-5-carboxylate
[0129] Dissolve 43.7g (0.2mol) of 2-chloro-4-fluoro-5-nitrobenzaldehyde oxime in 150ml of N,N-dimethylformamide, raise the temperature to 30°C, and add 32g (0.24 mol) NCS to form a light yellow solution, which was kept at 35° C. for 1 hour. Cool down to room temperature, add 300ml of dichloromethane, then wash twice with 1N hydr...
Embodiment 2
[0136] The preparation of embodiment 2 compound 5
[0137] Referring to the preparation of compound 6 in Example 1, the ethyl methacrylate added in the (2nd) step was replaced with methyl methacrylate. Oil. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm): 1.72(s,3H), 1.83(m,4H), 2.43(m,4H), 3.40(d,1H), 3.96(s,3H), 4.01(d,1H), 7.38( d,1H), 7.70(d,1H).
Embodiment 3
[0138] The preparation of embodiment 3 compound 4
[0139] 8g (0.019mol) of compound 6 was added to 20ml of ethanol, 1.52g (0.038mol) of sodium hydroxide aqueous solution was added, and the reaction was stirred at room temperature for 4 hours. The completion of the reaction was monitored by TLC, poured into water, adjusted to pH 3 with 1N hydrochloric acid, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain 7.2 g of light yellow oil.
[0140] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm):1.69(s,3H),1.85(m,4H),2.44(m,4H),3.41(d,1H),3.88(d,1H),7.35(d,1H),7.69( d, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com