Heterozygosis alpha spiral swine antibacterial peptide, and preparation method and application thereof
An antibacterial peptide and helical technology, which is applied in the field of hybrid α helical porcine antibacterial peptide and its preparation, can solve the problem of low antibacterial activity of the antibacterial peptide, and achieve high antibacterial activity, improved cell selectivity, and low cytotoxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Design and synthesis of hybrid α-helical porcine antimicrobial peptide sequence
[0017] The antimicrobial peptide RI16 was obtained by intercepting the first 16 amino acid residues at the N-terminal of the porcine antimicrobial peptide PMAP-36, and the antimicrobial peptide PRW4 was obtained by replacing the 7th and 11th amino acids with tryptophan, and then PRW4 was combined with α-helix The first 15 amino acids of the type antibacterial peptide Fowlicidin-2 were hybridized to obtain the antibacterial peptide PR-FO, and the carboxyl terminal of PR-FO was amidated to increase a positive charge and increase the stability of the peptide. The amino acid sequence of the antimicrobial peptide is shown in Table 1 Show.
[0018] Amino acid sequence and molecular weight of table 1 antimicrobial peptide
[0019]
[0020] Using a peptide synthesizer, the above-mentioned antimicrobial peptides were synthesized by solid-phase synthesis.
Embodiment 2
[0021] Destruction of the bacterial cell membrane of embodiment 2
[0022] 1 Destruction of the outer membrane by antimicrobial peptides
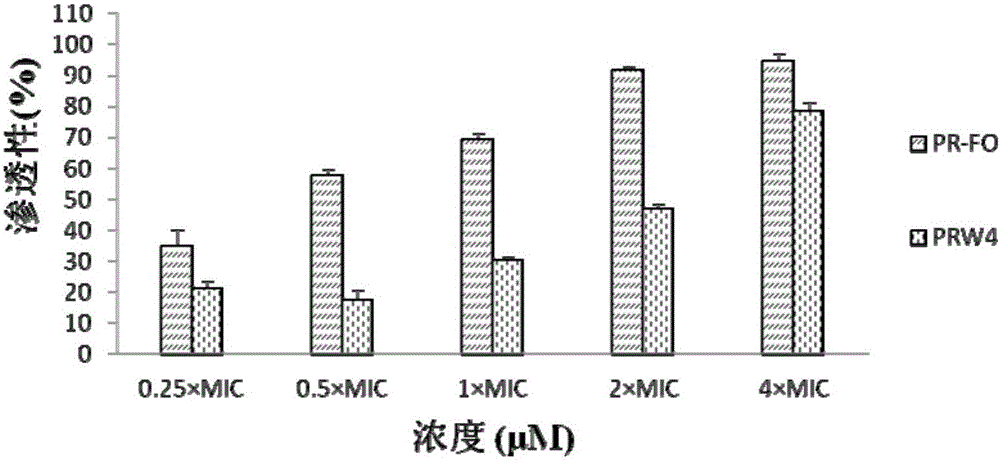
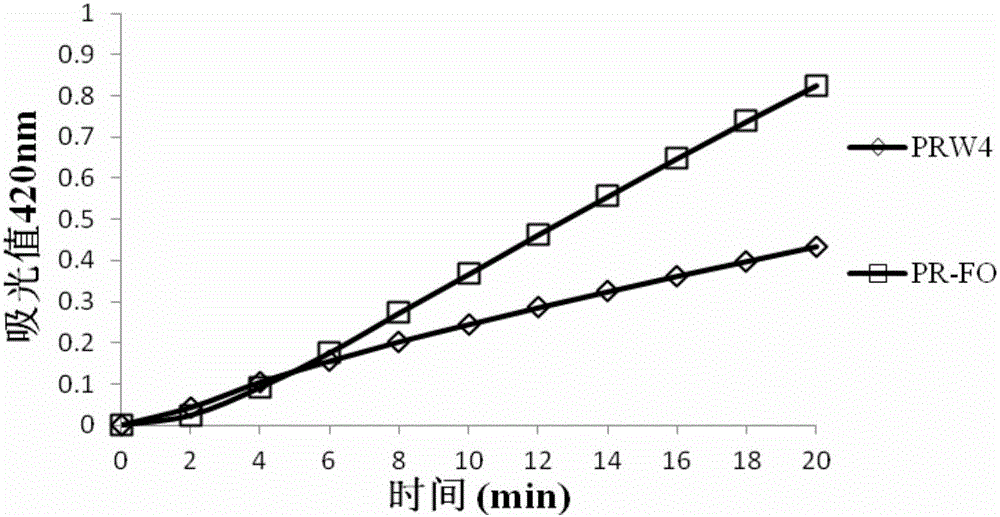
[0023] Escherichia coli was cultured to the logarithmic growth phase, centrifuged and collected, washed with 5 mM HEPES buffer solution (5 mM glucose, pH 7.4) and suspended in the buffer solution. Take 2mL of bacterial solution and put it in a quartz cuvette, and add 1mM NPN solution to mix with it, so that the final concentration of NPN is 10μM. Use F-4500 fluorescence spectrophotometer to detect the fluorescence intensity (excitation 350nm emission 420nm), add different concentrations of antimicrobial peptides, and detect the change of fluorescence intensity. The result is as figure 1 shown. Depend on figure 1 It can be seen that both PRW4 and PR-FO have a certain degree of permeabilization of the outer membrane, but the permeation intensity of PR-FO is stronger, indicating that the destruction effect of antimicrobial peptides on the ...
Embodiment 3
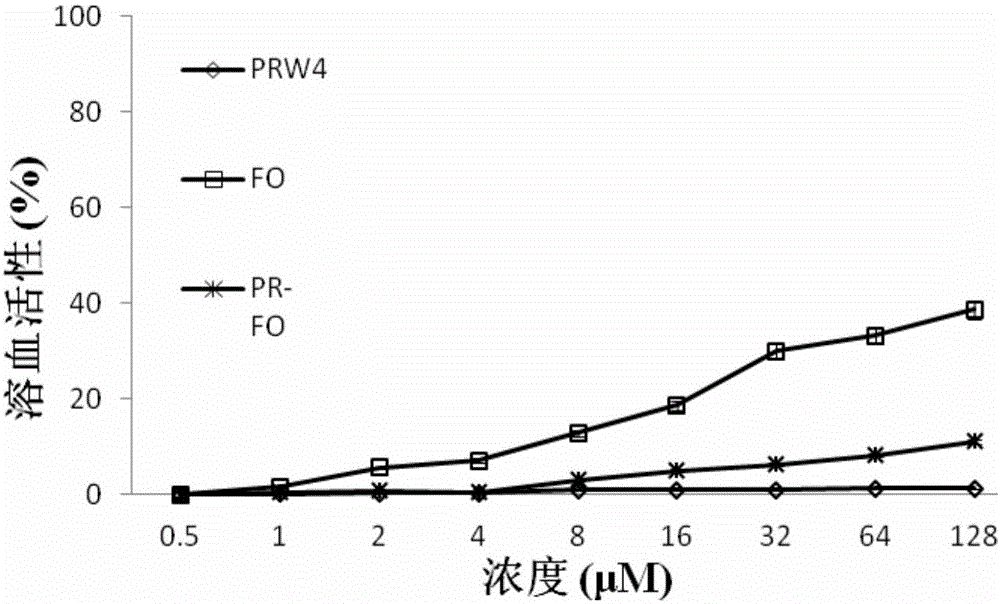
[0026] Bacteriostatic activity and hemolytic activity of embodiment 3 antimicrobial peptides
[0027] 1 Bacteriostatic activity of antimicrobial peptides
[0028] The antimicrobial peptides PRW4, FO and PR-FO were prepared as stock solution with a certain concentration for use. The minimum inhibitory concentrations of several antimicrobial peptides were determined by the broth microdilution method. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 individual / mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Incubate at a constant temperature of 37°C for 20 hours, and the minimum inhib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com