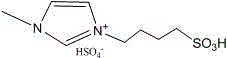Method for catalyzing cellulose hydrolysis by sulfonic acid functionalized ionic liquid composite system
A technology of sulfonic acid functionalization and ionic liquid, which is applied in the field of catalysis to achieve the effect of improving selectivity and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Mix 0.5g microcrystalline cellulose (MCC), 2.0g 1-(4-sulfobutyl)-3-methylimidazole bisulfate, 1mL H 2 O, 8mL of methyl isobutyl ketone was added to a 100mL lined glass tube, placed in a stainless steel autoclave, and heated to 150 o After C, electromagnetically stir, react under its own pressure (2.5atm) for 300min, then cool down to room temperature, filter, wash the reacted solution with water and absolute ethanol; collect the water phase to analyze the content of total reducing sugar after the reaction; the collected residue is in 100 Dry at ℃ for 8 hours, calculate the conversion rate of cellulose; collect the aqueous phase, organic phase and ethanol phase respectively, and analyze the content of the products 5-hydroxymethylfurfural (5-HMF), levulinic acid (LA) and furfural (Furfural) Yield.
Embodiment 2
[0016] Mix 0.5g microcrystalline cellulose (MCC), 2.0g 1-(4-sulfobutyl)-3-methylimidazole bisulfate, 1mL0.2mol / LMnCl 2 Aqueous solution, 8mL methyl isobutyl ketone into 100mL lined glass tube, placed in a stainless steel autoclave, to be heated to 150 o After C, electromagnetically stir, react under its own pressure (2.5atm) for 300min, then cool down to room temperature, filter, wash the reacted solution with water and absolute ethanol; collect the water phase to analyze the content of total reducing sugar after the reaction; the collected residue is in 100 Dry at ℃ for 8 hours, calculate the conversion rate of cellulose; collect the aqueous phase, organic phase and ethanol phase respectively, and analyze the yields of the products 5-HMF, LA and Furfural.
Embodiment 3~6
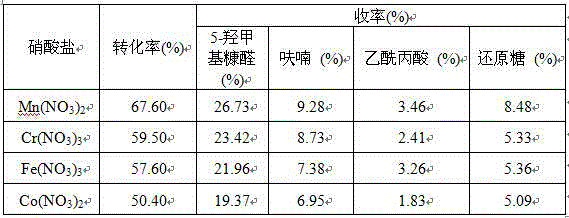
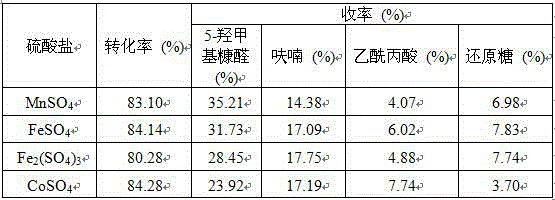
[0018] Other process conditions and experimental steps are with implementation example 2, but add different metal salt solutions (FeCl 3 , FeCl 2 , CoCl 2 , ZnCl 2 ) as co-catalyst, the results are shown in Table 1.
[0019] Table 1 The influence of different kinds of metal chlorides on the hydrolysis of MCC
[0020]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com