A kind of alkaline pectinase secretion enhanced bacterial strain and its application
A pectinase and enhanced technology, applied in the field of genetic engineering, can solve the problems of restricting the industrial production of alkaline pectinase, and the yield of alkaline pectinase cannot be further improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Construction and identification of recombinant bacteria
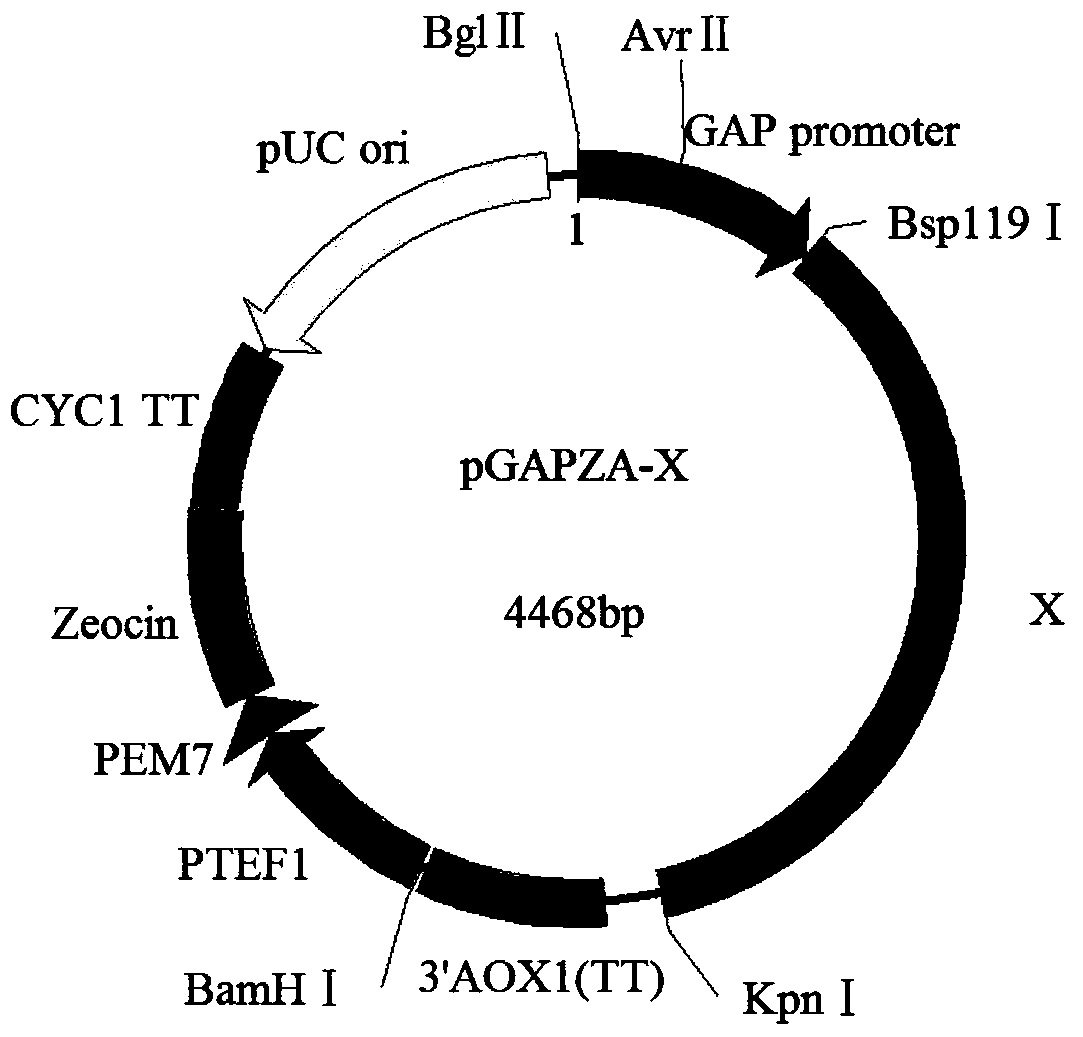
[0025] Extract Pichia pastoris GS115 RNA, reverse transcribe it into cDNA, use the cDNA as a template, design primers, and obtain UPR-related genes (HAC1, ERO1, SLY1, BMH2, GCN4, UBC1, HRD1, SSO2, SEC53, BIP, PDI , GSH2), and clone it into the expression vector pGAPZA to obtain the recombinant plasmid pGAPZA-X (see attached figure 1 , X represents HAC1, ERO1, SLY1, BMH2, GCN4, UBC1, HRD1, SSO2, SEC53, BIP, PDI or GSH2), the recombinant vector was transformed into Pichiapastoris GS115-pPIC9K-PGL, and the co-expression recombinant strain PichiapastorisGS115-pPIC9K was obtained after screening and identification -PGL / pGAPZA-X.
[0026] The Pichia pastoris GS115-pPIC9K-PGL expressing the recombinant alkaline pectinase is that the alkaline pectinase gene whose nucleotide sequence is shown in SEQ ID NO.1 is connected to the expression vector pPIC9K, and then transformed to Pichia pastoris host strain GS...
Embodiment 2
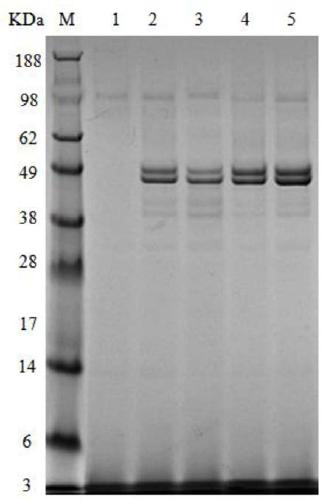
[0031] Example 2: Enzyme activity assay and protein electrophoresis of co-expressed genetically engineered strains
[0032] Cultivation method: After seed activation, the strain was inoculated into the basic fermentation medium YPD, cultured at 30°C and 220rpm for 14h, then transferred to the optimized growth medium BMGY and cultured at 30°C and 220rpm for 24h, and then the strain Transfer to the induction medium BMMY at 23°C, 220rpm and add 1.5% methanol every 24h to induce the expression of alkaline pectinase.
[0033] The enzyme activity measurement conditions are: the fermentation broth is centrifuged at 8000rpm for 10 minutes, extracellular PGL is contained in the fermentation supernatant, and a certain amount is taken for detection. PGL reaction system: glycine-NaOH buffer containing 0.2% polygalacturonic acid (substrate) (0.2mol L -1 , 0.44mmol·L -1 CaCl 2 , pH9.4) 2mL, the sample to be tested was 20μL, and the inactive enzyme solution was used as the blank control. ...
Embodiment 3
[0035] Embodiment 3: the purification of alkaline pectinase
[0036] Centrifuge the recombinant bacterial fermentation broth at 8000r / min for 20min, take the supernatant, add ammonium sulfate for gradient salting-out, collect 30-50% ammonium sulfate precipitated part by low-temperature centrifugation, and dissolve the salted-out precipitated enzyme in glycine-sodium hydroxide buffer Solution (pH7.5), dialyzed with 20mmol / L glycine-sodium hydroxide buffer solution for 24h. The supernatant obtained by centrifugation was further separated and purified by cation exchange chromatography.
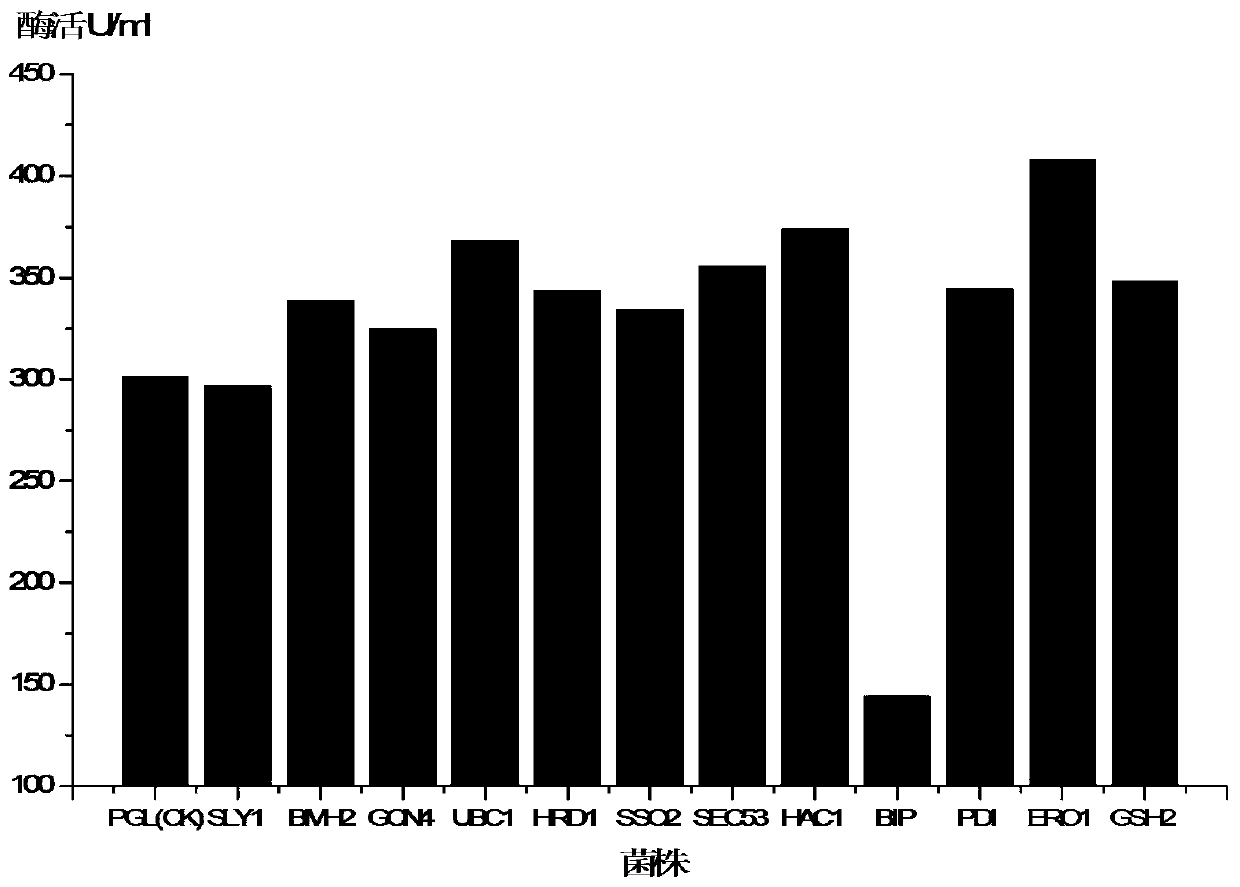
[0037] Compared with the starting strain Pichia pastoris GS115-pPIC9K-PGL before the co-expression of UPR-related genes, the enzyme activity was 301.32 U / ml after 96 hours of shake-flask induced fermentation, and the secretion-enhanced recombinant strain Pichia pastoris GS115-PGL / pGAPZA-HAC1 enzyme in the present invention The activity of 373.94U / ml increased by 24.1%, the activity of Pichia pas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com