Bacillus licheniformis high-temperature alpha-amylase mutant and application thereof
A technology of Bacillus licheniformis and amylase, which is applied in the fields of enzyme engineering and genetic engineering, can solve the problems of reduced benefits and increased production costs, and achieves the effects of reducing the use of saccharifying enzymes and reducing production costs
Active Publication Date: 2016-07-27
GUANGXI UNIV
View PDF7 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Since the main components of the syrup obtained by liquefying starch with high temperature resistant α-amylase are dextrin and oligosaccharides, and the glucose content is usually only 30%. A large number of saccharification enzymes and prolonging the saccharification time can obtain high-conversion glucose syrup, which increases the production cost and reduces the benefit
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0017] 1. Construction of mutant enzymes
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
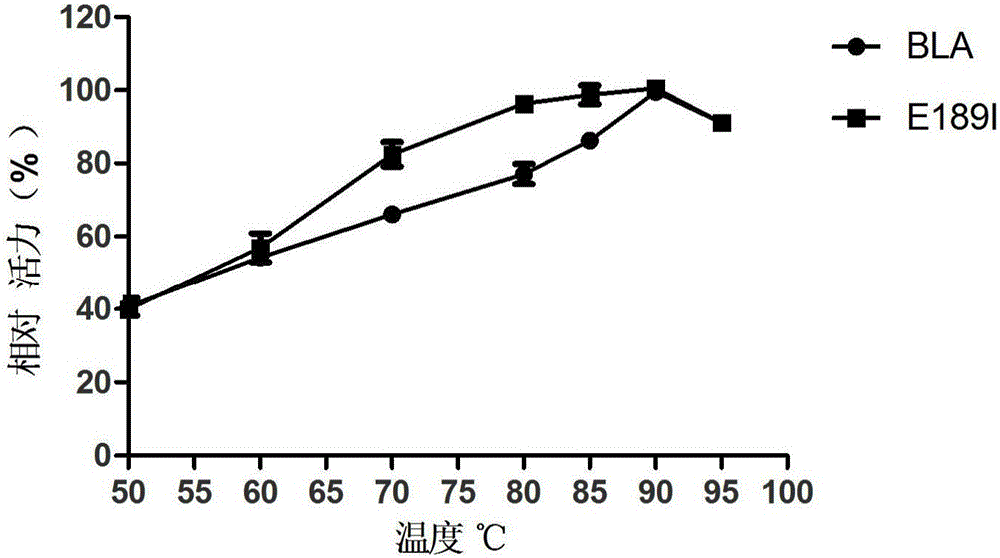
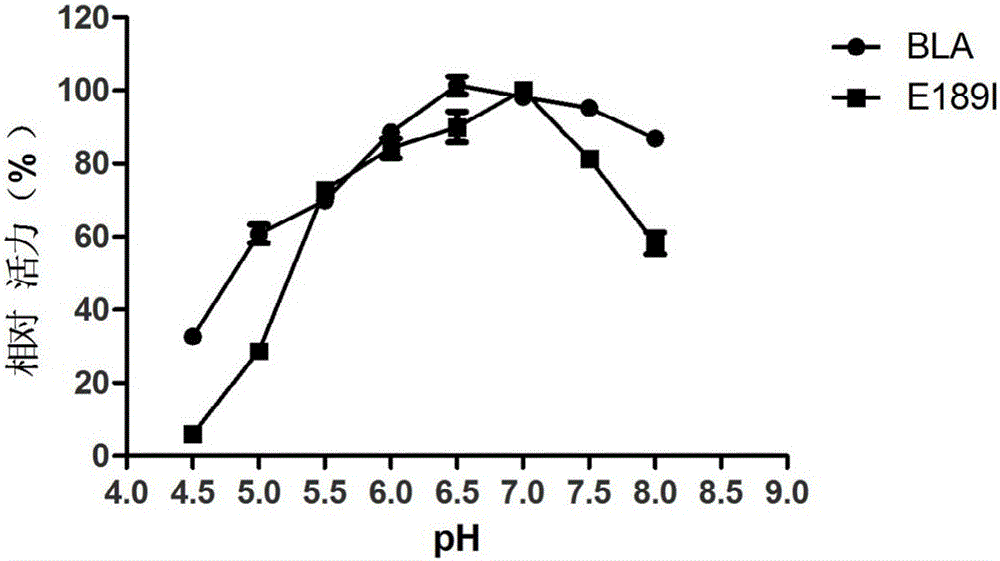
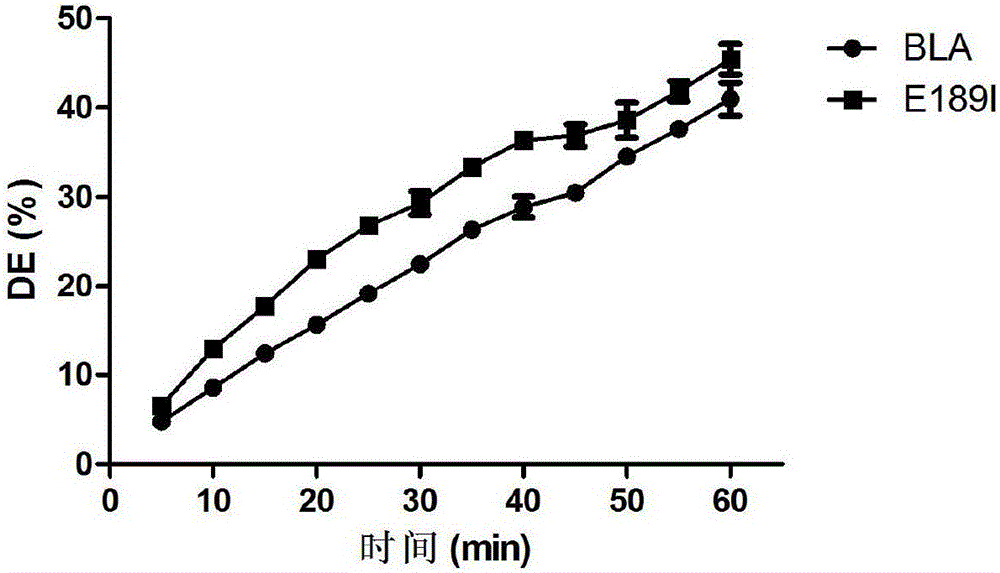
Provided are a bacillus licheniformis high-temperature alpha-amylase mutant and application thereof.A nucleotide sequence of the high-temperature alpha-amylase mutant is shown as SEQ ID NO.1, and an amino acid sequence of the high-temperature alpha-amylase mutant is shown as SEQ ID NO.2.The mutant is obtained by conducting protein engineering improvement and selection on high-temperature alpha-amylase from bacillus licheniformis, the glucose content in a product of hydrolyzed starch can be improved and the dextrin and oligosaccharide content can be reduced by applying mutant enzyme in the hydrolyzed starch, the glucose content in syrup obtained through separate starch hydrolysis is 72%, the using amount of saccharifying enzyme in the follow-up further glucose production can be decreased, and reduction of the production costs is promoted.
Description
technical field [0001] The invention relates to the fields of enzyme engineering and genetic engineering, in particular to a bacillus licheniformis high-temperature alpha-amylase mutant. Background technique [0002] Bacillus licheniformis (Bacillus licheniformis) high temperature-resistant α-amylase BLA has high temperature resistance and high activity, and can randomly hydrolyze starch, glycogen and the α-1.4 glucoside key in the interior of its degradation products to make the viscosity of gelatinous starch solution drop rapidly. The instantaneous temperature in the liquefaction process reaches 105-110°C, and the starch can still be effectively hydrolyzed and the liquefied syrup obtained from the liquefied starch is of high quality, which is widely used in the starch liquefaction process of food fermentation industries such as alcohol, beer, organic acid and starch sugar. , and is currently the most widely used high-temperature α-amylase. But its disadvantages are also o...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C12N9/28C12P19/14C12P19/12C12P19/02C12P19/00C12R1/10
CPCC12N9/2417C12P19/00C12P19/02C12P19/12C12P19/14C12Y302/01001
Inventor 韦宇拓汤宏赤刘明瑞杜丽琴
Owner GUANGXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com