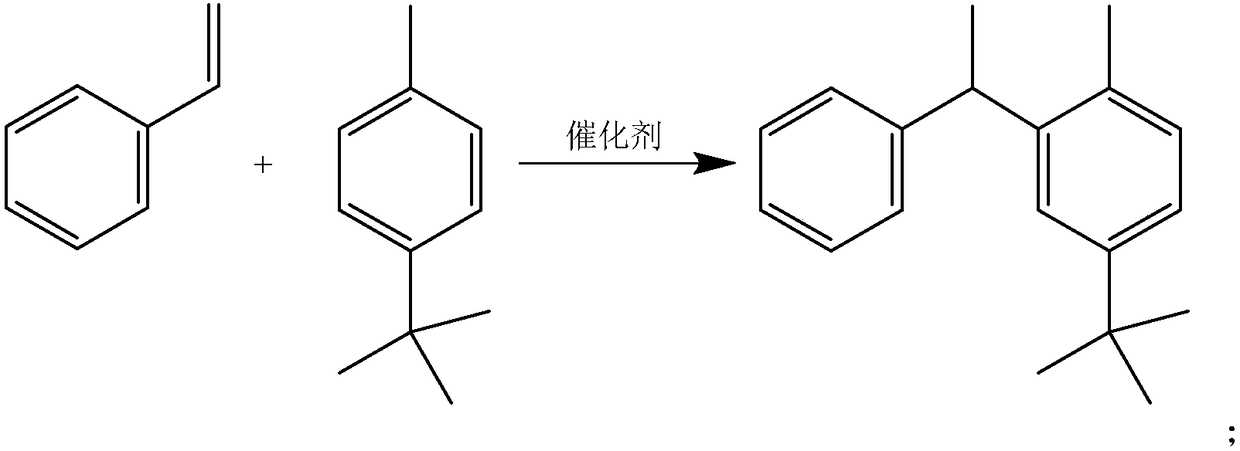
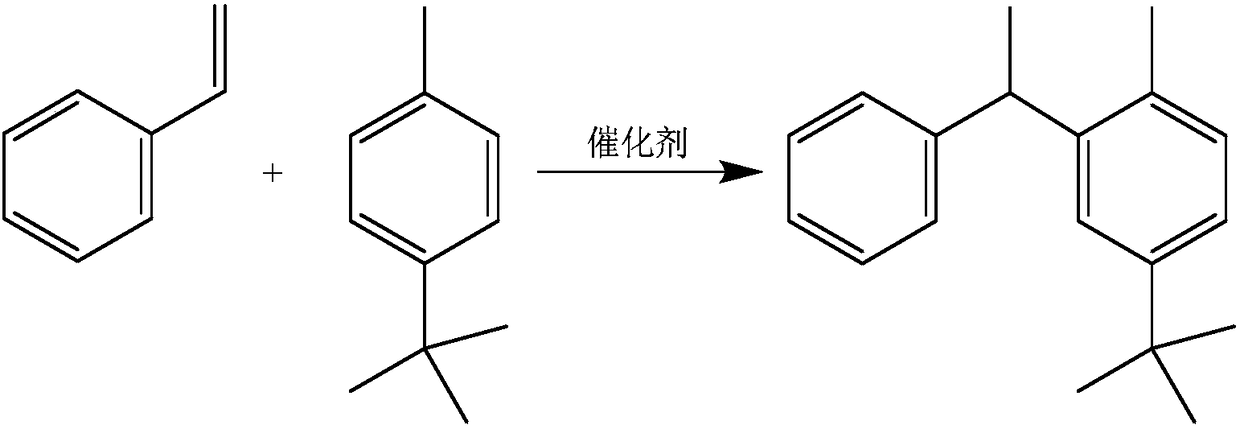
1-phenyl-1-(p-tert-butyltolyl)ethane and its synthesis method
A technology of p-tert-butyltoluene and synthesis method, which is applied in the application field of high-temperature heat transfer oil, can solve the problems of needing alkaline water washing or hydrochloric acid decomposition, difficult control of reaction temperature, difficult industrial production, etc., and achieves low freezing point, difficult decomposition, side effects The effect of less product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A kind of synthetic method of 1-phenyl-1-(p-tert-butyltolyl) ethane, comprises the steps:
[0020] 1) P-tert-butyltoluene is added into the flask, and a solid acidic catalyst is added under stirring conditions, and the solid acidic catalyst accounts for 2.5% of the total mass of the reaction solution. In the solid acid catalyst, the mass ratio of solid acid to bismuth p-toluenesulfonate is 1:1;
[0021] 2) Heating with a heating mantle, the temperature rose to 125°C, and slowly added styrene dropwise for 2 hours. In this example, the molar ratio of p-tert-butyltoluene:styrene=7.0:1;
[0022] 3) After the dropwise addition, continue to stir for 10 minutes, cool it to below 10°C to fully separate the catalyst, filter and recover the catalyst;
[0023] 4) adding magnesium oxide and activated clay with a mass ratio of 1:10 to the filtrate, the total amount of magnesium oxide and activated clay accounts for 1-2% of the weight of the entire reaction solution. Heat to 80°C a...
Embodiment 2
[0029] A kind of synthetic method of 1-phenyl-1-(p-tert-butyltolyl) ethane, comprises the steps:
[0030] 1) P-tert-butyltoluene is added into the flask, and a solid acidic catalyst is added under stirring conditions, and the solid acidic catalyst accounts for 4.5% of the total mass of the reaction solution. In the solid acid catalyst, the mass ratio of solid acid to bismuth p-toluenesulfonate is 1:1;
[0031] 2) Use a heating mantle to heat, the temperature rises to 150° C., and slowly add styrene dropwise for 2 hours. In this example, the molar ratio of p-tert-butyltoluene:styrene=5.5:1;
[0032] 3) After the dropwise addition, continue to stir for 10 minutes, cool it to below 10°C to fully separate the catalyst, filter and recover the catalyst;
[0033] 4) adding magnesium oxide and activated clay with a mass ratio of 1:10 to the filtrate, the total amount of magnesium oxide and activated clay accounts for 1-2% of the weight of the entire reaction solution. Heat to 80°C a...
Embodiment 3
[0039] A kind of synthetic method of 1-phenyl-1-(p-tert-butyltolyl) ethane, comprises the steps:
[0040] 1) add p-tert-butyl toluene in the flask, add solid acidic catalyst under the condition of stirring, solid acidic catalyst accounts for 3% of reaction solution gross mass. In the solid acid catalyst, the mass ratio of solid acid to bismuth p-toluenesulfonate is 1:1;
[0041] 2) Use a heating mantle to heat, the temperature rises to 100° C., and slowly add styrene dropwise for 2 hours. In this embodiment, the molar ratio of p-tert-butyltoluene:styrene=6:1;
[0042] 3) After the dropwise addition, continue to stir for 10 minutes, cool it to below 10°C to fully separate the catalyst, filter and recover the catalyst;
[0043] 4) adding magnesium oxide and activated clay with a mass ratio of 1:10 to the filtrate, the total amount of magnesium oxide and activated clay accounts for 1-2% of the weight of the entire reaction solution. Heat to 80°C and stir for more than 1.5h, coo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com