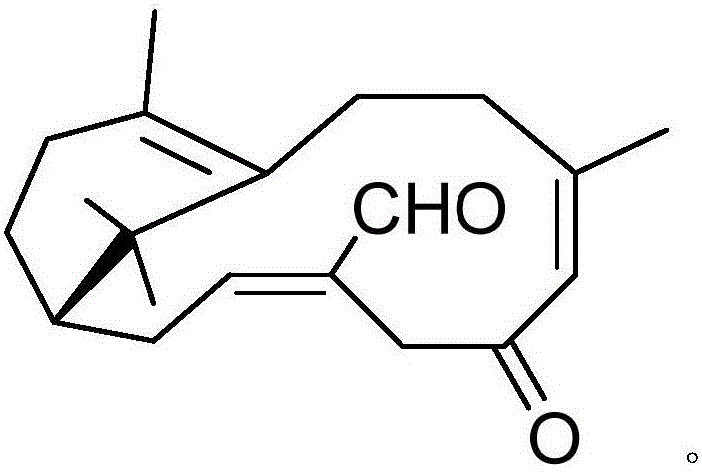
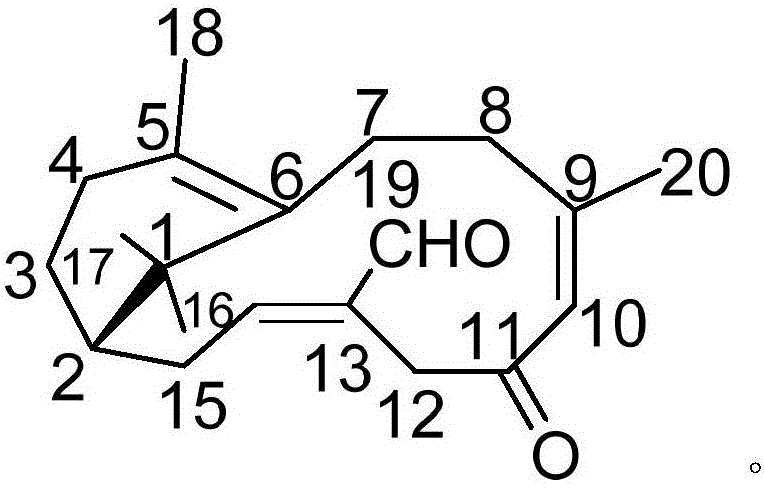
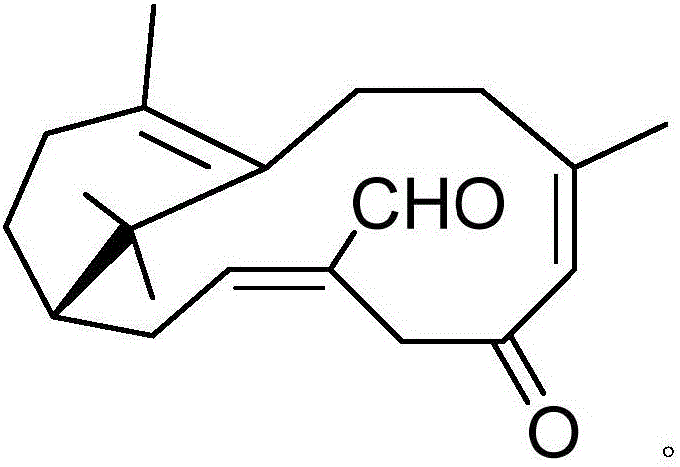
Pharmaceutical composition containing acipimox and medical application of pharmaceutical composition
A composition and drug technology, applied in the field of pharmaceutical compositions of acyclolimus, can solve problems such as no reports on the relevance of pharmaceutical compositions, and achieve the effects of outstanding substantive characteristics, remarkable progress, and improved neuroprotective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Compound (I) Separation Preparation and Structure Confirmation
[0019] Sources of reagents: ethanol, petroleum ether, ethyl acetate, n-butanol, and dichloromethane were of analytical grade, purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Methanol, of analytical grade, were purchased from Jiangsu Hanbang Chemical Reagent Co., Ltd.
[0020] Separation method: (a) crush the dried mint leaves (5kg), extract with 75% ethanol under heat reflux (20L × 3 times), combine the extracts, concentrate until no alcohol smell (4L), and then use petroleum ether (4L× 3 times), ethyl acetate (4L × 3 times) and water-saturated n-butanol (4L × 3 times) were extracted to obtain petroleum ether extract, ethyl acetate extract and n-butanol extract respectively; (b) step (a) The ethyl acetate extract in (a) was removed with D101 type macroporous resin, first eluted with 8% ethanol for 6 column volumes, then with 70% ethanol for 8 column volumes, collected 70% eluate, and...
Embodiment 2
[0023] Example 2: Neuroprotection
[0024] 1. Materials and Instruments
[0025] The PC12 cell line was donated by the Experimental Center of Anhui University of Traditional Chinese Medicine. Aβ 1-40Protein was purchased from Sigma Company. Compound (I) is self-made, and the HPLC normalized purity is greater than 98%. DMEM / F12 medium (imported packaging was purchased from Beijing Thermo Fisher Biochemical Products Co., Ltd. NewbomCalfSerum was purchased from Beijing SolarbioScience&Technology Co., Ltd. MTT was purchased from Amresco, USA. KGI AnnexinV-FITC Cell Apoptosis Detection Kit was purchased from From Nanjing KGI Biotechnology Co., Ltd. Paraformaldehyde was purchased from China Pharmaceutical Group Chemical Reagent Co., Ltd. Bcl-2 polyclonal antibody was purchased from Wuhan Boster Biotechnology Co., Ltd. Bax polyclonal antibody was purchased from Beijing Boaosen Biotechnology Co., Ltd. Cyt-c polyclonal antibody was purchased from Wuhan Boster Biotechnology Co., Ltd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com