Preparation method of vinyl sulfone derivatives
A technology for vinyl sulfone and derivatives, which is applied in the field of preparation of polyethylene glycol vinyl sulfone derivatives, can solve the problems of low yield and difficult separation of polyethylene glycol, and achieve high conversion rate, fast reaction speed, The effect of good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
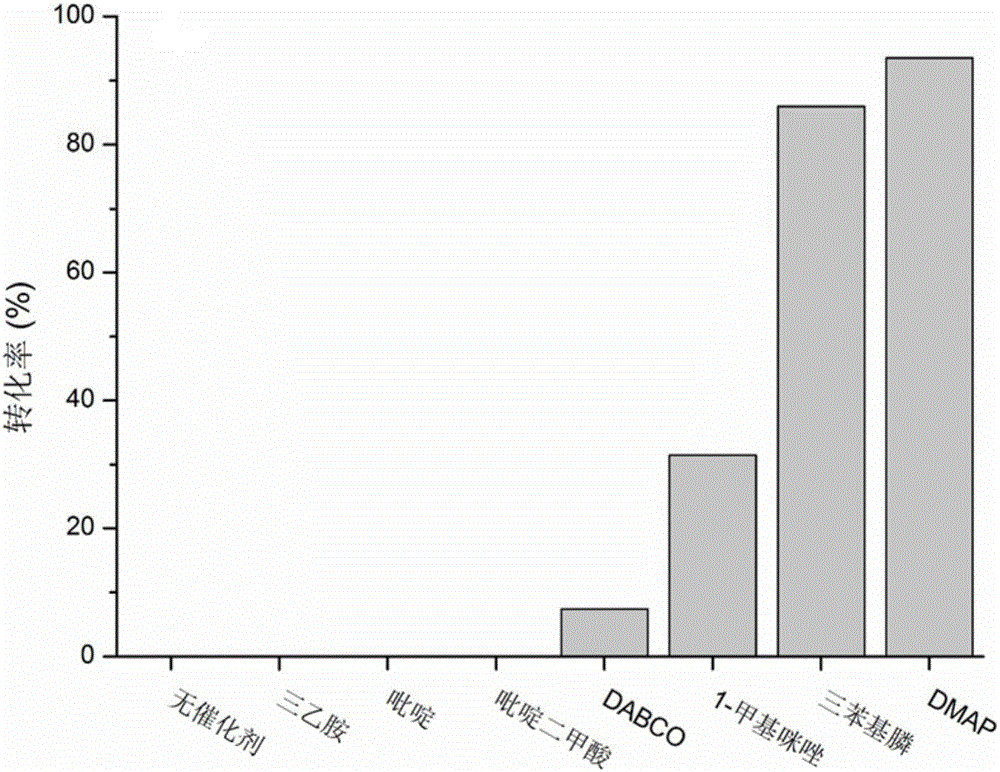
[0030] DMAP catalyzed the preparation of methyl tripolyethylene glycol vinyl sulfone derivatives (n=3):
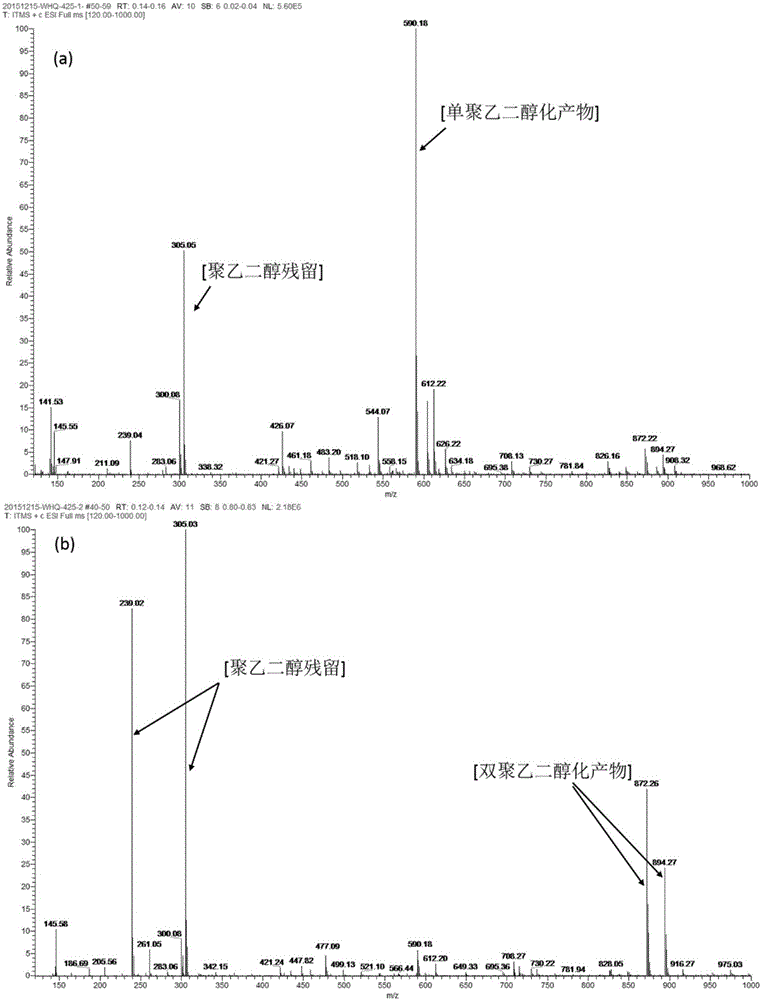
[0031] Take 1mL of methyl tripolyethylene glycol (Me-PEG 3 ) was dissolved in 4 mL of divinyl sulfone (DVS), 60 mg of DMAP was added, reacted for 2 hours at room temperature, and purified by column chromatography to obtain methyl tripolyethylene glycol vinyl sulfone derivatives (93% yield). 1 HNMR (400MHz,D 2 O): δ6.63(dd,1H,SO 2 CH=CH 2 ),6.42(d,1H,SO 2 CH=CH 2 ),6.18(d,1H,SO 2 CH=CH 2 ), 3.91(t,2H,SO 2 CH 2 CH 2 ),3.57(t,2H,SO 2 CH 2 CH 2 OCH 2 ),3.48(t,2H,SO 2 CH 2 CH 2 ),3.33(m,3H,CH 3 O),3.63(m,10H,othersCH 2 ).
Embodiment 2
[0033] Triphenylphosphine catalyzed the preparation of methyl tripolyethylene glycol vinyl sulfone derivatives (n=3):
[0034] Take 1mL of methyl tripolyethylene glycol (Me-PEG 3 ) was dissolved in 4mL divinyl sulfone (DVS), and 125 mg triphenylphosphine was added, reacted for 2 hours at room temperature, purified by column chromatography to obtain methyl tripolyethylene glycol vinyl sulfone derivative (yield was 76%) . 1 HNMR (400MHz,D 2 O): δ6.63(dd,1H,SO 2 CH=CH 2 ),6.42(d,1H,SO 2 CH=CH 2 ),6.18(d,1H,SO 2 CH=CH 2 ), 3.91(t,2H,SO 2 CH 2 CH 2 ),3.57(t,2H,SO 2 CH 2 CH 2 OCH 2 ),3.48(t,2H,SO 2 CH 2 CH 2 ),3.33(m,3H,CH 3O),3.63(m,10H,othersCH 2 ).
Embodiment 3
[0036] 1-Methylimidazole catalyzes the preparation of methyl tripolyethylene glycol vinyl sulfone derivatives (n=3):
[0037] Take 1mL of methyl tripolyethylene glycol (Me-PEG 3 ) was dissolved in 4mL divinylsulfone (DVS), added 40mg1-methylimidazole, reacted for 2 hours at room temperature, and purified by column chromatography to obtain methyl tripolyethylene glycol vinylsulfone derivatives (yield: 32%) . 1 HNMR (400MHz,D 2 O): δ6.63(dd,1H,SO 2 CH=CH 2 ),6.42(d,1H,SO 2 CH=CH 2 ),6.18(d,1H,SO 2 CH=CH 2 ), 3.91(t,2H,SO 2 CH 2 CH 2 ),3.57(t,2H,SO 2 CH 2 CH 2 OCH 2 ),3.48(t,2H,SO 2 CH 2 CH 2 ),3.33(m,3H,CH 3 O),3.63(m,10H,othersCH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com