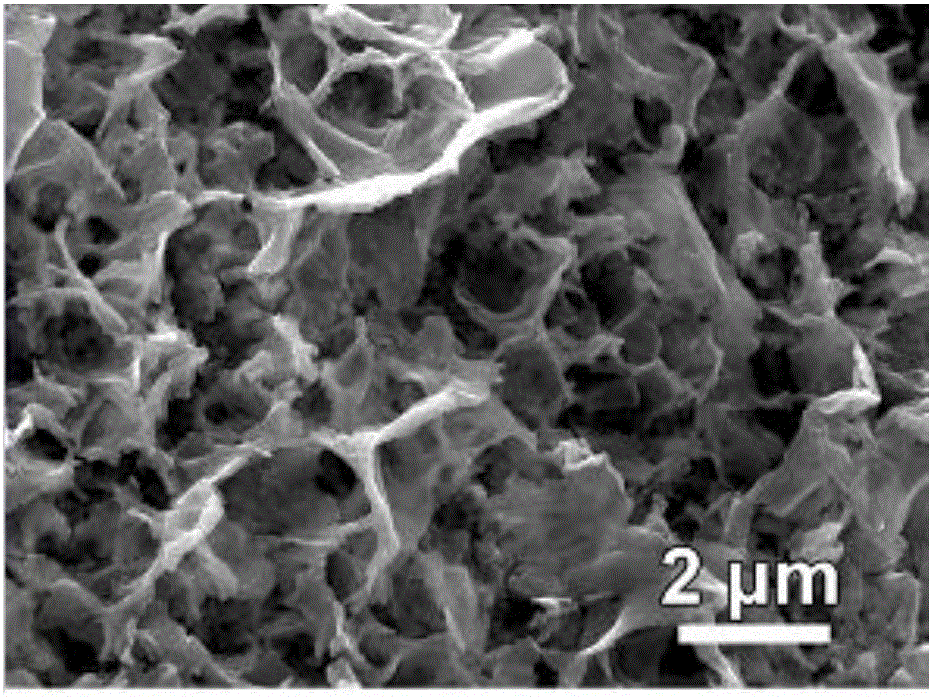
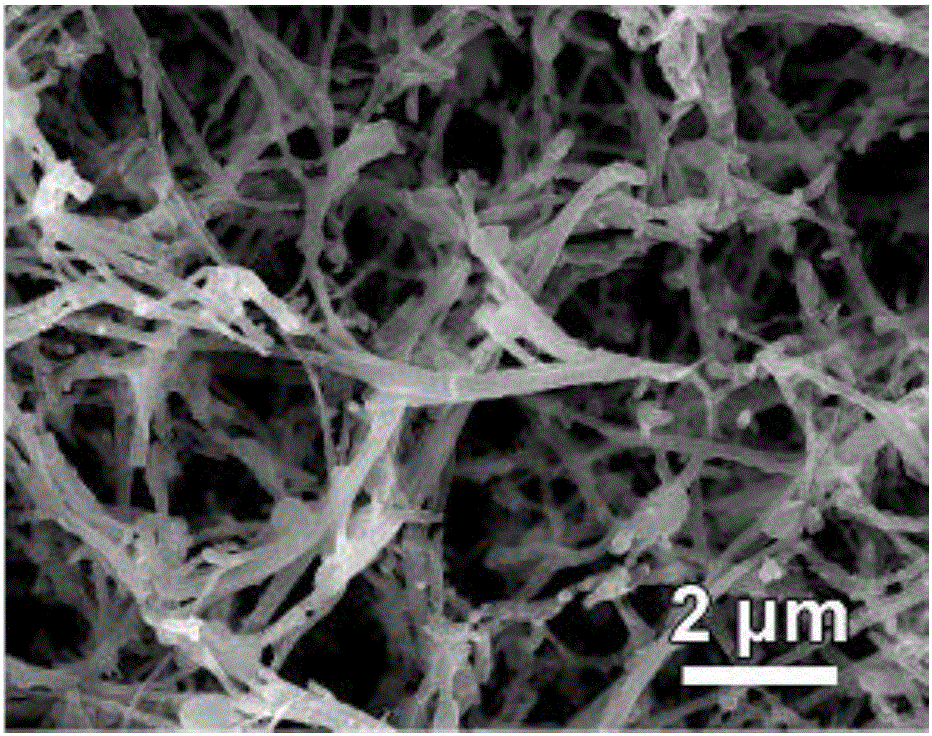
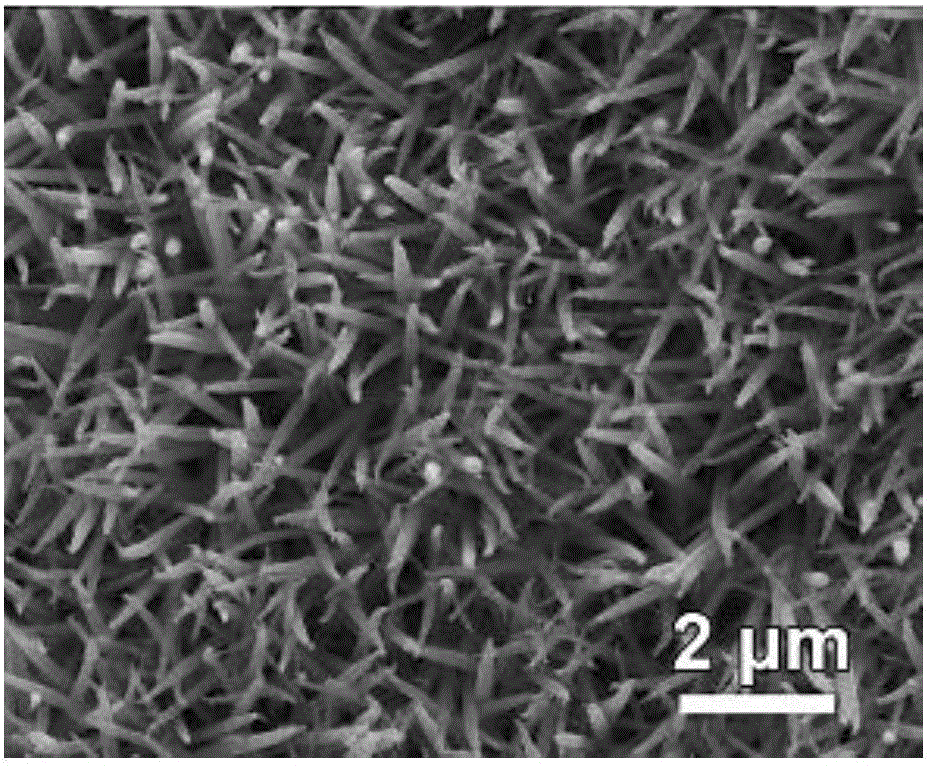
Surface treatment method for improving electro-catalysis hydrogen production performance
A surface treatment and electrocatalysis technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of reduced electrocatalytic hydrogen evolution performance and poor stability, and achieve the effects of excellent electrocatalytic performance, low cost and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A surface treatment method for improving electrocatalytic hydrogen production performance, comprising the following steps:
[0038] S1: Preparation of precursors by hydrothermal growth method:
[0039] S11, first dissolve 9mmol nickel chloride and 18mmol hexamethylenetetramine in 60mL deionized water to make a solution;
[0040] S12, then vertically fix the foamed nickel sheet cleaned with 3M hydrochloric acid and remove the surface oxide layer in the 100mL polytetrafluoroethylene liner equipped with the solution of step S11, and seal it with an autoclave;
[0041] S13. Put the polytetrafluoroethylene liner sealed in step S12 into an oven at 120° C. for 8 hours, and finally wash and dry the reaction product to obtain a nickel salt nanosheet precursor, that is, the nickel salt nanosheet precursor is long with nickel Nickel Foam with Salt Nanosheets.
[0042] S2: Prepare phosphorus-containing nanosheets by the precursor prepared in step S1:
[0043] S21. Using 0.9 g so...
Embodiment 2
[0049] The difference from Example 1 is:
[0050] In step S11, first dissolve 9 mmol of cobalt chloride and 18 mmol of urea in 60 mL of deionized water to make a solution; and the material obtained in S13 is a cobalt salt nanowire precursor, that is, the cobalt salt nanowire precursor is a cobalt salt nanowire long nickel foam;
[0051] In step S21, the sodium dihydrogen hypophosphite powder and the tailored cobalt salt nanowire precursor are put into the quartz tube, and the CoP nanowire is obtained through step S23;
[0052] In step S3, the CoP nanowire is used as the working electrode (i.e., the CoP nanowire electrocatalytic electrode), and the surface roughness and surface active sites of the CoP nanowire increase after CV cycle scanning, which improves the electrocatalytic production of the CoP nanowire. Hydrogen properties.
[0053] Draw the electrocatalytic activity diagrams before and after the CoP nanowire CV cycle treatment provided by this embodiment: as Figure ...
Embodiment 3
[0055] The difference from Example 1 is:
[0056] In step S11, first dissolve 3mmol nickel chloride, 6mmol cobalt chloride and 18mmol urea in 60mL deionized water to make a solution; and the material obtained in S13 is the precursor of nickel-cobalt salt nanowires, that is, the precursor of nickel-cobalt salt nanowires It is nickel foam with nickel-cobalt salt nanowires;
[0057] In step S21, the sodium dihydrogen hypophosphite powder and the trimmed nickel-cobalt salt nanowire precursor are put into the quartz tube, and the obtained NiCoP nanowire is obtained through step S23;
[0058] In step S3, the NiCoP nanowire is used as the working electrode (i.e., the NiCoP nanowire electrocatalytic electrode), and the surface roughness and surface active sites of the NiCoP nanowire increase after CV cycle scanning, which improves the electrocatalytic production of the NiCoP nanowire. Hydrogen properties.
[0059] Draw the electrocatalytic activity figure before and after the NiCoP ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com