Staphylococcus aureus vaccine and preparation method thereof
A staphylococcus, golden yellow technology, applied in the field of microbial vaccines, can solve problems such as narrow scope of application, poor immunogenicity, and no coverage of toxic proteins, and achieve the effect of reducing mortality and preserving immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of staphylococcus aureus vaccine, comprising the following steps:
[0038] (1) Inoculate the bacterial solution of Staphylococcus aureus standard strain ATCC 25923 on a blood plate, and incubate at 37°C for 24 hours;
[0039] (2) Pick 1 colony, inoculate it into high-pressure sterilized tryptone broth, shake and culture at 37°C for 18h to OD 600 0.6-0.8, the speed is 220rpm;
[0040] (3) Centrifuge at 3000rpm for 10min, discard the supernatant, resuspend the pellet with sterile PBS and centrifuge again, repeat 3 times to remove the medium and metabolites, and resuspend the pellet obtained after the last centrifugation with sterile PBS , so that its concentration is 1×10 9 / ml;
[0041] (4) Place the bacterial liquid on ice, and carry out radiation treatment with a gamma ray generating device, the radiation intensity is 10Gy / min, the radiation time is 10h, and the total dose is 6kGy;
[0042] (5) Aspirate 10 μl of the irradiated bacterial solution ...
Embodiment 2
[0046] Verification of immune protection of Staphylococcus aureus vaccine
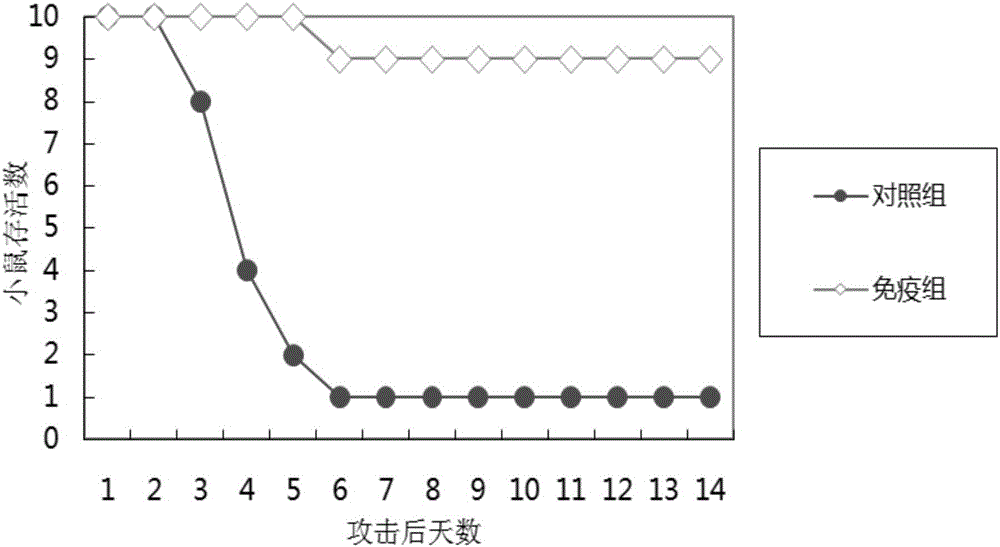
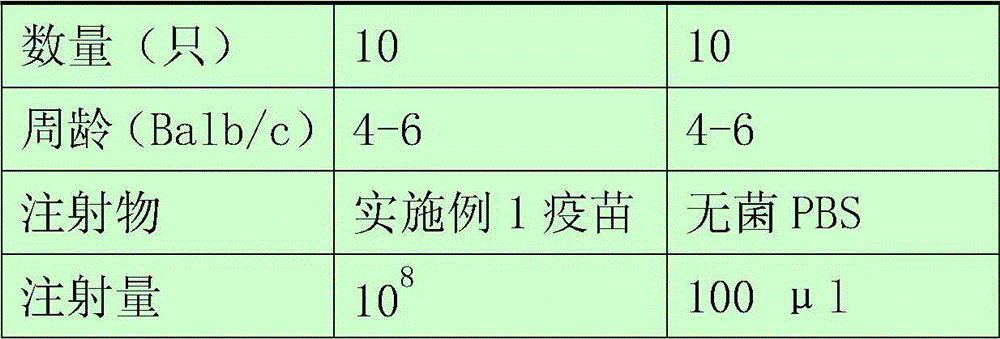
[0047] Detect the protectiveness of Staphylococcus aureus vaccine in 4-6 week old Balb / c mice, each 10 of immunization group and control group, immunization group and control group are as shown in table 1:
[0048]
[0049]
[0050] Table 1
[0051] 2 weeks after the initial immunization, use the same dose in Table 1 for the second immunization injection; 3 weeks after the initial immunization, use the same dose in Table 1 for the last immunization injection; 2 weeks after the last immunization, intraperitoneally inject 0.5ml of lethal dose of Staphylococcus aureus ATCC 25923 to the mice , the number of bacteria is about 1×10 7 . The morbidity and death of the mice were observed, and the survival rates of the mice in the immunized group and the control group were counted after 2 weeks. Specific results such as figure 1 shown.
[0052] Depend on figure 1 It can be seen that the survival rate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com