Medical product with active ingredient coating and method for producing the same
A technology of active material coating and active material, which is applied in the field of medical products for the treatment of vascular diseases, and can solve the problems of low adhesion on the surface of stents or balloons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 10 mg of paclitaxel in solid form was provided by drying a stock solution of paclitaxel in ethyl acetate having defined concentrations. Paclitaxel was absorbed into a mixture of ethyl acetate and different contents of dimethylsulfoxide (DMSO) and it was checked whether a clear solution formed after 72 hours at room temperature.
[0067] In another group (Ansatz) the maximum solubility of paclitaxel in DMSO was determined to be 400 mg / ml.
[0068] Solution experiments showed that the composition of the coating solution consisting of 10 mg paclitaxel in 200 μl ethyl acetate and 12 μl DMSO already gave the desired transparent solution. The active substance content in this solution is about 833 mg / ml DMSO, thus approximately 2.1 times the maximum solubility of paclitaxel in DMSO.
Embodiment 2
[0070] A coating solution of 10 mg paclitaxel in 200 μl ethyl acetate and 22 μl DMSO was prepared. In this coating solution, the active substance content was 454 mg paclitaxel / ml DMSO, which is about 13.5% higher than the maximum solubility of paclitaxel in DMSO. Therefore, the solution contained an insufficient amount of DMSO to completely dissolve paclitaxel (10 mg) contained in a solvent mixture of 200 μl ethyl acetate and 22 μl DMSO.
[0071] The coating solution was applied to a stainless steel panel and left at room temperature under normal pressure until the solvent evaporated completely. A paclitaxel coating with a crystal structure was obtained.
[0072] The coating experiment was repeated using the solution from example 1 (10 mg of active substance in 200 μl of ethyl acetate and 12 μl of DMSO), again obtaining a crystalline coating of paclitaxel.
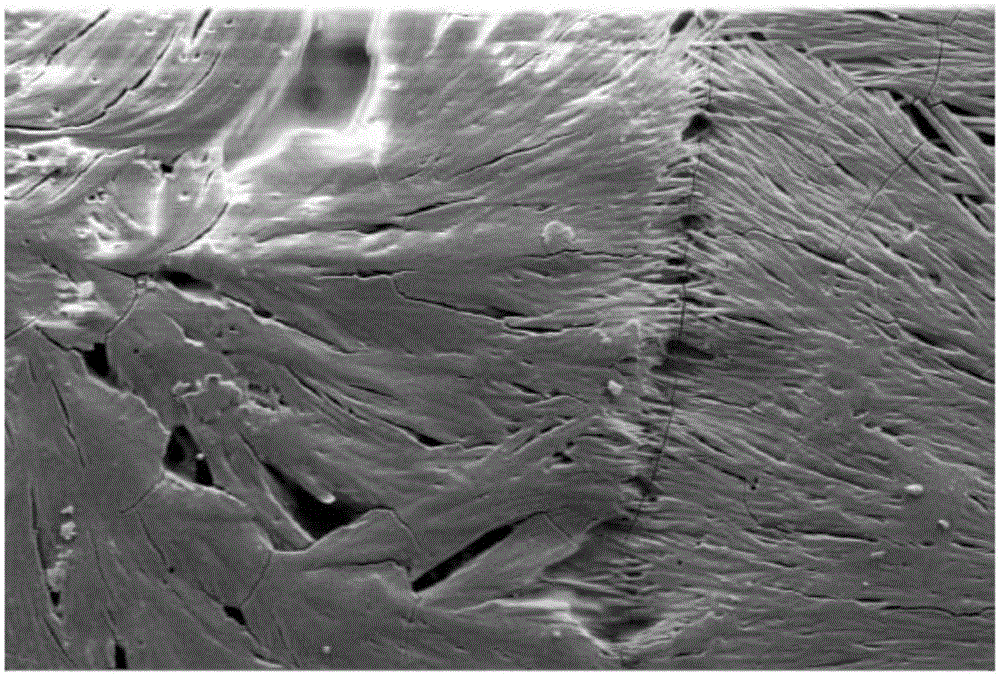
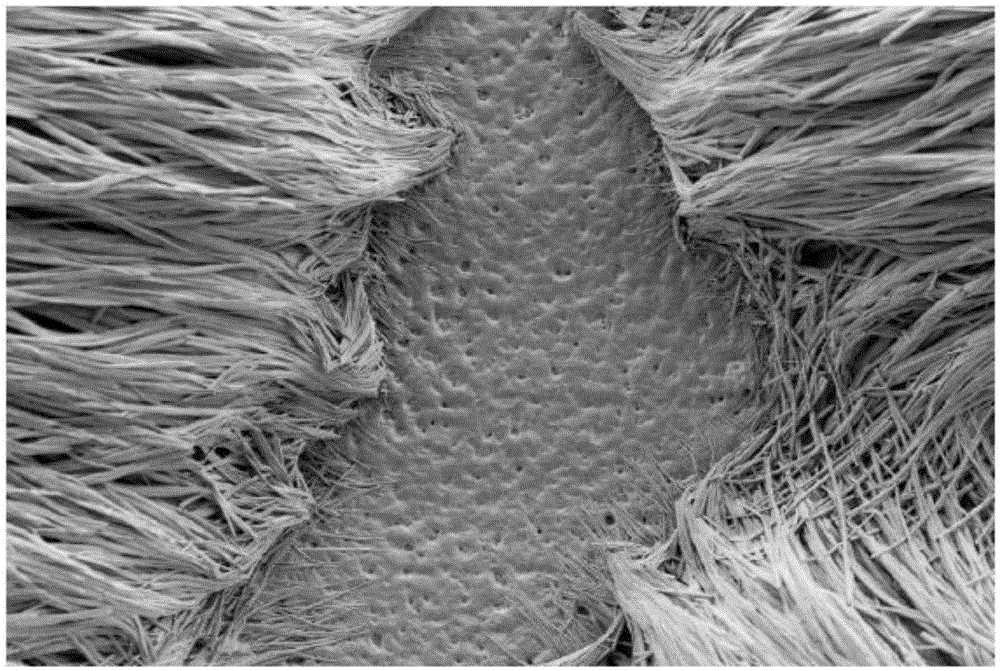
[0073] exist figure 1 In , an electron micrograph of the coating obtained from the coating solution of Example 2 at m...
Embodiment 3
[0076] 10 mg paclitaxel was dissolved in 2000 μl ethyl acetate and 22 μl DMSO. Unlike Example 2, the amount of the volatile first solvent was increased tenfold.
[0077] The coating solution thus obtained was applied to a stainless steel plate and left at room temperature under normal pressure until the solvent evaporated completely. A paclitaxel coating with a crystalline structure was also obtained. It can thus be shown that the second solvent remains in the coating even in the case of intensive dilution with the volatile first solvent and that the active substance recrystallizes into crystalline form after removal of the first solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com