Harmaline oxazoline compound and preparation method and application thereof
A technology for alkali oxazoline and camelina, which is applied in the field of camelina oxazoline compounds and preparation thereof, and achieves the effects of good growth and development regulating activity, simple synthesis process and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
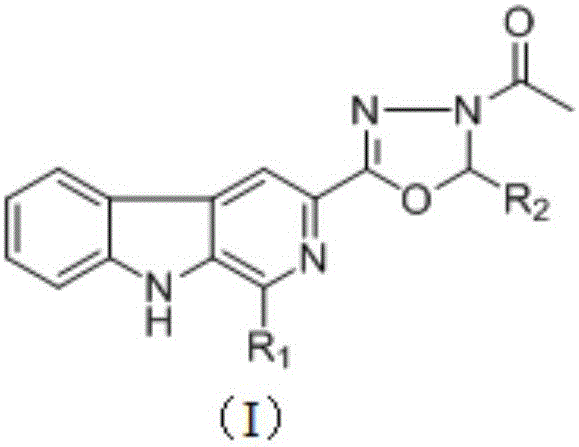
[0048] Example 1: Synthesis of 1-methyl-3-[5-(2-o-hydroxyphenyl)-3-N-acetyl-1,3,4-oxazoline]-β-carboline (7a)
[0049] 1. Preparation method
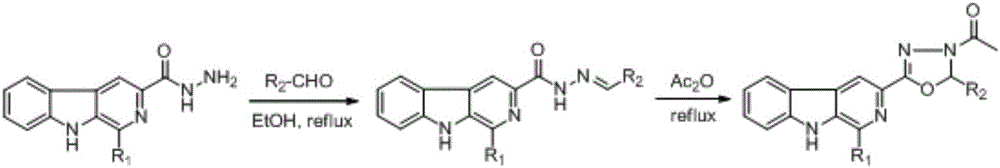
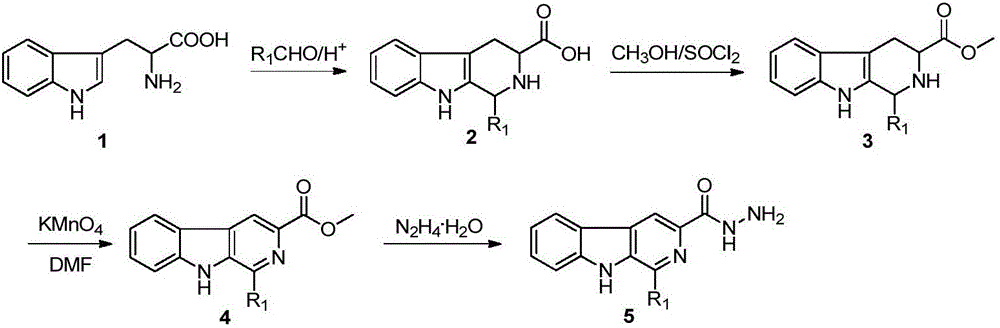
[0050] (1) Step A: Synthesis of 1-methyl-β-carboline-3-o-hydroxybenzoylhydrazone
[0051] Take 0.5g of 1-methyl-β-carboline-3-hydrazide in a 50mL single-necked bottle, add 10mL of absolute ethanol, add 0.27g of o-hydroxybenzaldehyde into the bottle while stirring, add 1mL of glacial acetic acid at the same time, heat to reflux After 2 hours, most of the solvent was removed by rotary evaporation, and the remaining suspension was recrystallized with dilute ethanol, filtered, and dried to obtain 1-methyl-β-carboline-3-o-hydroxybenzoylhydrazone with a yield of 93%;
[0052] (2) Step B: 1-methyl-3-[5-(2-o-hydroxyphenyl)-3-N-acetyl-1,3,4-oxazoline]-β-carboline (7a) Synthesis
[0053] Weigh 0.8g of 1-methyl-β-carboline-3-o-hydroxybenzoylhydrazone and add it to a 50mL round bottom flask installed on a magnetic stirrer, add 10mL of acetic anh...
Embodiment 2
[0056] Example 2: Synthesis of 1-phenyl-3-[5-(2-o-hydroxyphenyl)-3-N-acetyl-1,3,4-oxazoline]-β-carboline (7b)
[0057] 1. Preparation method
[0058] The operation is the same as in Example 1, except that in step A, 1-methyl-β-carboline-3-hydrazide is replaced by 1-phenyl-β-carboline-3-hydrazide.
[0059] 2. The product testing data is as follows:
[0060] Yield: 67%; 1 H NMR (600MHz, DMSO-d 6 )δ: 11.91 (s, 1H, 9-NH), 8.95 (s, 1H, 2′-H), 8.76 (s, 1H, 4-H), 8.44 (d, J=7.8Hz, 1H, 5- H), 8.19(d, J=18.6Hz, 1H, 8-H), 8.00(t, J=8.4Hz, 2H, Ar-H), 7.72(m, 1H, Ar-H), 7.68(t, J=12.6Hz, 1H, A-H), 7.63(t, J=15.6Hz, 2H, Ar-H), 7.60(d, J=16.2Hz, 1H, 7-H), 7.56(d, J=17.4Hz ,1H,Ar-H),7.34(d,J=13.6Hz,1H,Ar-H),7.31(t,J=7.2Hz,1H,6-H),6.80(d,J=3.6Hz,1H ,Ar-H),6.53(s,1H,Ar-H),1.91(s,3H,3′-COCH 3 ). 13 C NMR (151MHz, DMSO-d 6 )δ:169.56,169.44,160.76,148.96,148.53,143.11,141.45,138.07,138.00,135.22,131.16,130.92,130.25,129.98,129.65,128.88,127.77,126.60,126.44,123.74,122.47,122.25,121.01,12...
Embodiment 3
[0061] Example 3: 1-p-chlorophenyl-3-[5-(2-o-hydroxyphenyl)-3-N-acetyl-1,3,4-oxazoline]-β-carboline (7c) Synthesis
[0062] 1. Preparation method
[0063] The operation is the same as in Example 1, except that in step A, 1-methyl-β-carboline-3-hydrazide is replaced by 1-p-chlorophenyl-β-carboline-3-hydrazide.
[0064] 2. The product testing data is as follows:
[0065] Yield: 70%; 1 H NMR (600MHz, DMSO-d 6)δ: 11.97(s,1H,9-NH),11.91(s,1H,OH),9.00(s,1H,2′-H),8.69(s,1H,4-H),8.48(d, J=7.9Hz, 1H, 5-H), 8.24(d, J=8.5Hz, 2H, Ar-H), 7.90(dd, J=7.8, 1.4Hz, 1H, Ar-H), 7.73(d, J=8.5Hz, 2H, Ar-H), 7.70(d, J=8.1Hz, 1H, 8-H), 7.63(t, J=7.6Hz, 1H, 7-H), 7.49(td, J= 7.9,1.6Hz,1H,Ar-H),7.40(t,J=7.3Hz,1H,Ar-H),7.35(t,J=7.5Hz,1H,6-H),7.22(d,J= 7.3Hz,1H,Ar-H),2.46(s,3H,3′-COCH 3 ). 13 C NMR (151MHz, DMSO-d 6 )δ:169.49,161.34,156.97,148.89,143.79,141.57,139.68,138.99,136.06,134.51,133.78,130.91,130.33,130.05,128.82,128.79,128.10,126.77,126.32,125.86,123.49,122.14,121.09,120.40 , 114.44,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com