Disulfide bond cross-linked C3 polypeptide supermolecule helical polymer and preparation method thereof
A technology of supramolecular polymers and disulfide bonds, which is applied in the field of C3 polypeptide supramolecular helical polymers and its preparation, can solve the problems of limited application and lack of functionalization, and achieve enhanced stability, high thermal stability and solvent resistance , Easier self-assembly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
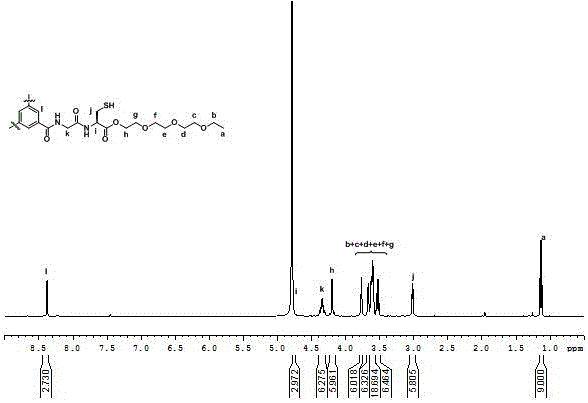
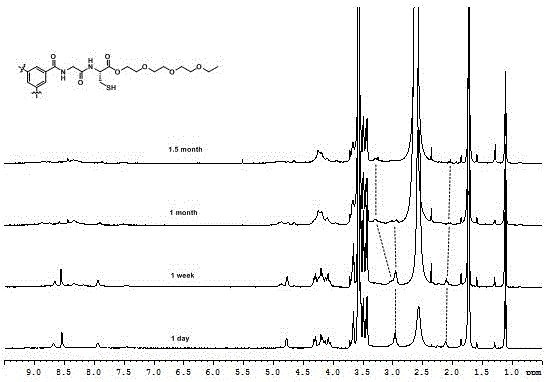
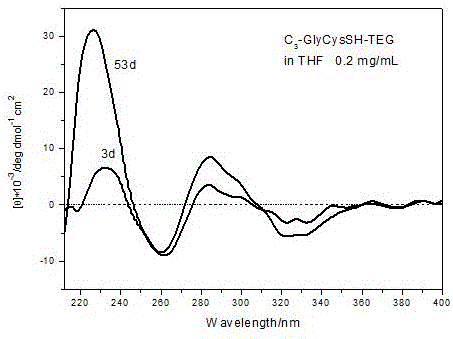
[0044] a. Synthesis of dipeptide Boc-GlyCys(Acm)-TEG: Add 1.02 g of Cys(Acm)-TEG and 0.35 g of Boc-Gly-OH to a 100 mL round bottom flask, then add 0.29 g of HOBt and 1.56 g of DiEA, replace N 2 Afterwards, the reaction was carried out in an ice-salt bath for 12 h, and the crude product was respectively washed with saturated KHSO 4 solution and saturated Na 2 CO 3 Washed, dried and filtered over anhydrous magnesium sulfate, and purified by silica gel column chromatography to obtain 1.08 g of dipeptide Boc-GlyCys(Acm)-TEG with a yield of 81%.
[0045] b. Contains Acm protecting group C 3 -Synthesis of GlyCys(Acm)-TEG: Add 300 mg of the deprotected dipeptide GlyCys(Acm)-TEG to a 50 mL round bottom flask, add 500 mg of DiEA dropwise after DCM is dissolved, adjust the pH of the solution to greater than 11, and replace the N 2 Afterwards, 130 mg of pentafluorophenol active trimesic acid was added dropwise, and reacted at room temperature for 48 h. After the reaction was complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com