Detection method of ginsenoside in evodia decoction and application thereof
A detection method, ginsenoside technology, applied in Evodia decoction, the detection field of ginsenoside in vivo and in vitro, can solve the problems of poor UV absorption, low bioavailability, and poor absorption of saponins, and achieve simple and fast operation, Accurate and reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: chromatographic conditions: chromatographic column is Agilent ZORBAX SB-C18 column (4.6mm * 150mm, 5 μ m), mobile phase is the formic acid solution of methanol-0.1%, gradient elution (4min-58% methanol, 6min-90% methanol, 6.01min-58% methanol). The flow rate was 0.5mL·min-1, and the column temperature was 20°C.
[0020] Mass spectrometry conditions: ion injection voltage 5500V, source gas 1 at 310.275KPa, source gas 2 at 344.75KPa, curtain gas at 137.9KPa, source temperature at 500°C. The detection method is positive ion detection, the scanning method is selective reaction monitoring (MRM) method, and the ion pair (m / z) used for quantitative analysis: 823.6 / 203.1 (ginsenoside Rg 1 ), 969.7 / 789.0 (ginsenoside R e ), 1131.8 / 365.1 (ginsenoside Rb 1 ) and 823.6 / 789.5 (ginsenoside R d ).
Embodiment 2
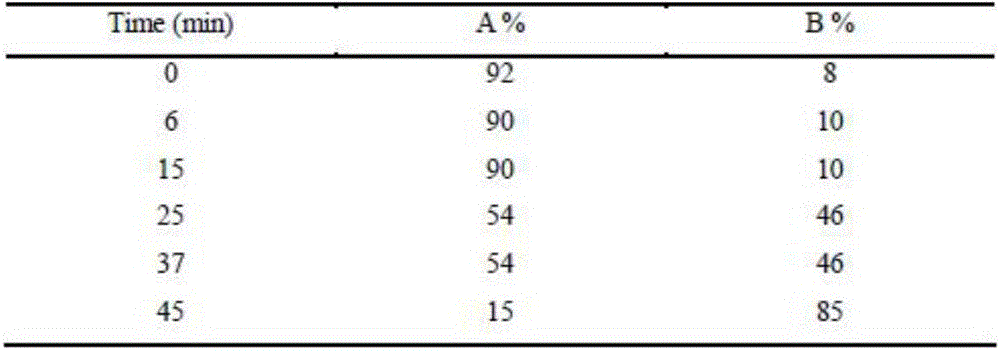
[0021] Example 2: Chromatographic conditions: chromatographic column is RESTEK Pinnacle-C18 column (50mm-2.1mm, 5 μm); column temperature: 20°C; flow rate is 200 μL / min; mobile phase is A water (volume fraction 0.5‰ formic acid), B acetonitrile (volume fraction 0.5‰ formic acid), gradient elution (0-in, mobile phase B volume fraction 20%-50%; 13-13.1min, mobile phase B volume fraction 50%-20%; 13.1-23min, mobile phase Phase B volume fraction 20%).
[0022] Mass spectrometry conditions: electrospray ESI ion source; curtain gas 10ps; atomization gas (GAS1) 40ps; heating auxiliary gas (GAS2) 40ps; collision gas CAD medium; spray voltage IS 5500V; atomization temperature 500°C ; The detection method is positive ion multiple ion reaction detection (MRM, the ion used for quantitative analysis is m / z R g 1832.8 → 643.6; R e 969.8 → 789.7; R b 11132.1 → 365.3.
Embodiment 3
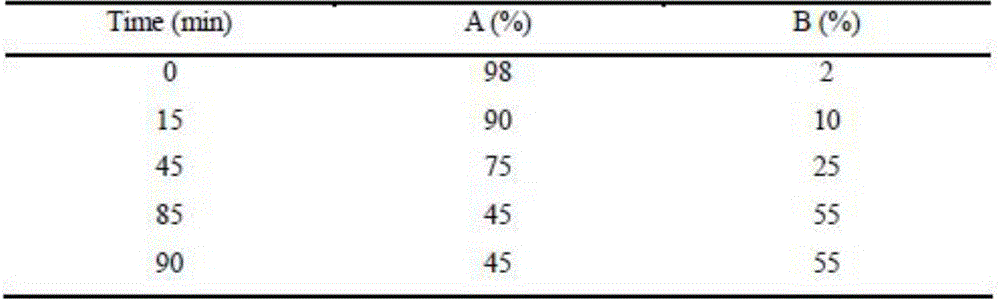
[0023] Embodiment 3: chromatographic conditions: chromatographic column: Apollo C18 chromatographic column (250 * 4.6mm, 5 μ m), mobile phase: A pump: 0.1% formic acid water; B pump: 0.1% formic acid acetonitrile, gradient program is as follows:
[0024]
[0025] Column temperature: 35°C, flow rate: 1.0ml / min (25% split into mass spectrometer), injection volume: 5μl.
[0026]Mass spectrometry conditions: first-level full scan conditions: ESI positive ion scanning, scanning mass range: 150-1000 (chloroform extraction site) and 150-1500 (n-butanol extraction site); capillary voltage: 2.8kV; cone voltage: 30V; Extraction voltage: 3.0V, lens voltage: 0.0V; source temperature: 120°C; desolvation gas flow rate and temperature are 600ml / min and 350°C, respectively; cone gas: 50ml / min. Under the above chromatographic conditions and mass spectrometry conditions, the samples from the chloroform extraction parts of Evodia rutaecarpa, the chloroform extraction parts of Evodia rutaecarp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com