Ring opening myrtle ketone analogue as well as preparation method and application thereof to antibacterial medicines
An antibacterial drug, the technology of aurinone, which is applied in antibacterial drugs, resistance to vector-borne diseases, and pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation method of open-ring myrtone analogue:
[0028] The synthesis process of the ring-opened myrtone analog of the present invention is as follows:
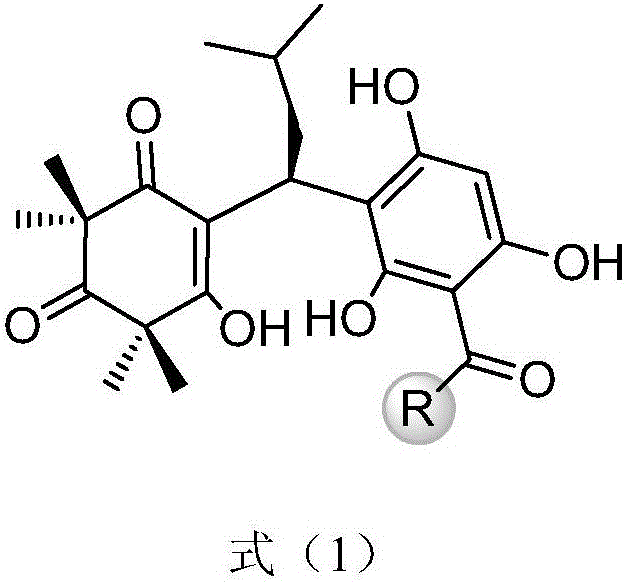
[0029]
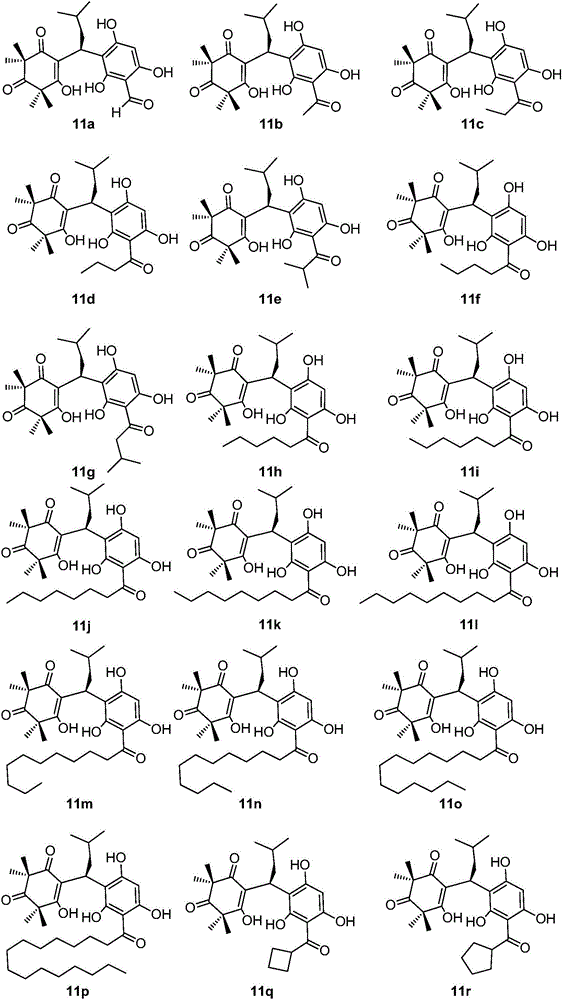
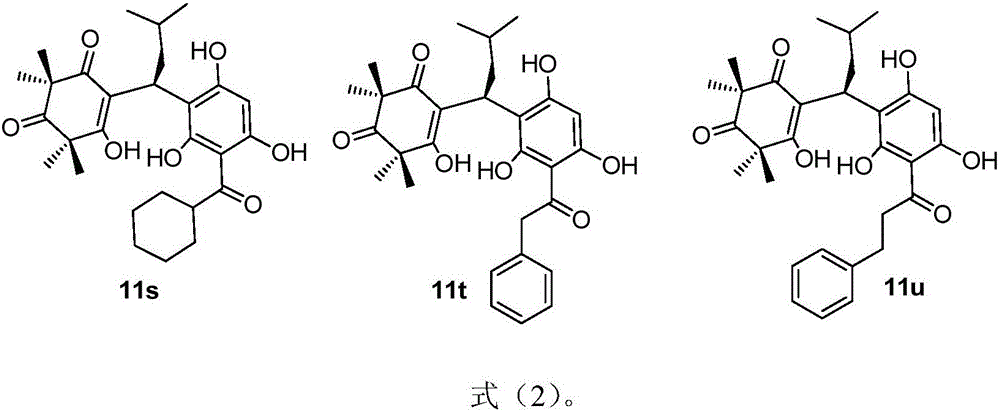
[0030] Use phloroglucinol and alkyl acid chloride under the catalysis of methanesulfonic acid or aluminum trichloride, obtain the precursor compound 6 in the formula (5) through Friedel-Crafts acylation reaction, wherein R is H, C 1 -C 15 Straight chain, branched chain and cycloalkyl or aromatic groups containing structures such as benzene rings; another precursor compound 10 can be carried out under alkaline conditions by using acetylphloroglucinol 6b as raw material to carry out C-methyl and then quickly obtained through protonic acid-catalyzed reverse Claisen condensation and Knoevenagel condensation; the obtained precursor compound 6 and compound 10 (α, β-unsaturated ketone) are further promoted to undergo Michael addition under basic conditions. reaction, the target product 11 ca...
Embodiment 2
[0114] Embodiment 2: the anti-MRSA activity evaluation of compound
[0115] In this embodiment, the minimum inhibitory concentration (MIC) of the anti-MRSA activity of the sample will be determined by the resazurin chromogenic method. In the test, the 96-well plate dilution titer technique will be used to simultaneously determine the minimum inhibitory concentration (MIC) of various substances. First, mix 7.5 mL of indicator solution (100 μg / mL resazurin aqueous solution) with 5 mL of the bacteria solution to be tested (10 8 CFU / mL) and mix well, and add 100 μL of mixed bacterial solution to all test wells in columns 1 to 8. Then add 100 μL of the DMSO solution (64 μg / mL) of the sample compound 11a-11u to be tested into each well of the first column in sequence, and after uniform mixing, take out 100 μL of the solution and transfer it to the corresponding well of the second column, and use the same Method for doubling dilutions to column 8. Finally, put the orifice plate wi...
Embodiment 3
[0120] Embodiment 3: the anti-SA activity evaluation of compound
[0121] In this embodiment, the minimum inhibitory concentration (MIC) of the anti-SA activity of the sample will be determined by the resazurin chromogenic method. In the test, the 96-well plate dilution titer technique will be used to simultaneously determine the minimum inhibitory concentration (MIC) of various substances. First, mix 7.5 mL of indicator solution (100 μg / mL resazurin aqueous solution) with 5 mL of the bacteria solution to be tested (10 8 CFU / mL) and mix well, and add 100 μL of mixed bacterial solution to all test wells in columns 1 to 8. Then add 100 μL of the DMSO solution (64 μg / mL) of the samples 11a-11u to be tested into the wells of the first column in sequence, and after uniform mixing, take out 100 μL of the solution and transfer it to the corresponding wells of the second column, and use the same Method doubling dilutions to column 8. Finally, put the orifice plate with the sample a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com