Thermal activation delayed fluorescence material containing 1, 10 phenanthroline unit and application of material
A technology for thermally activated delay and fluorescent materials, applied in luminescent materials, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as poor thermal stability, fast efficiency roll-off, high cost of electroluminescent device materials, etc. Problems, to achieve high energy utilization, high RISC rate, improve luminous efficiency and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
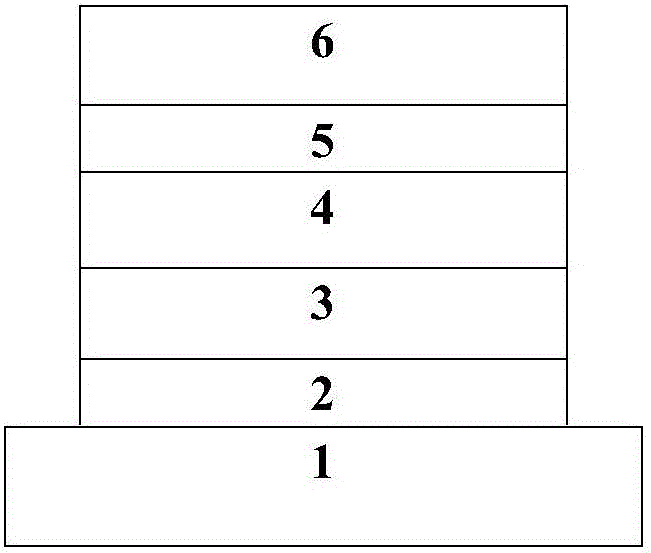
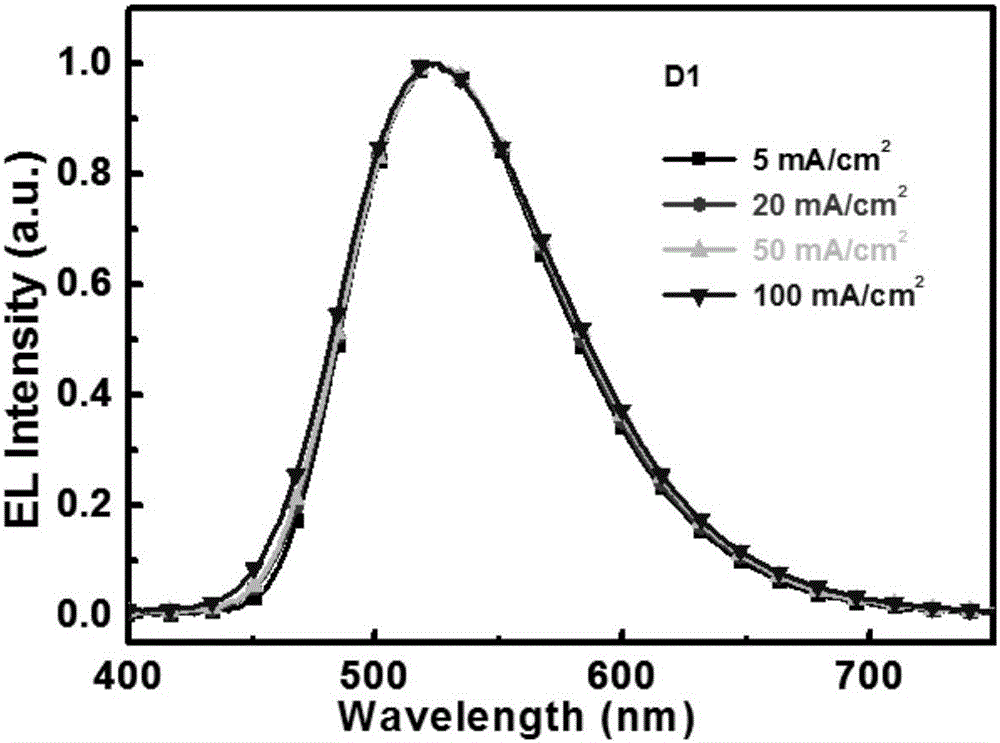
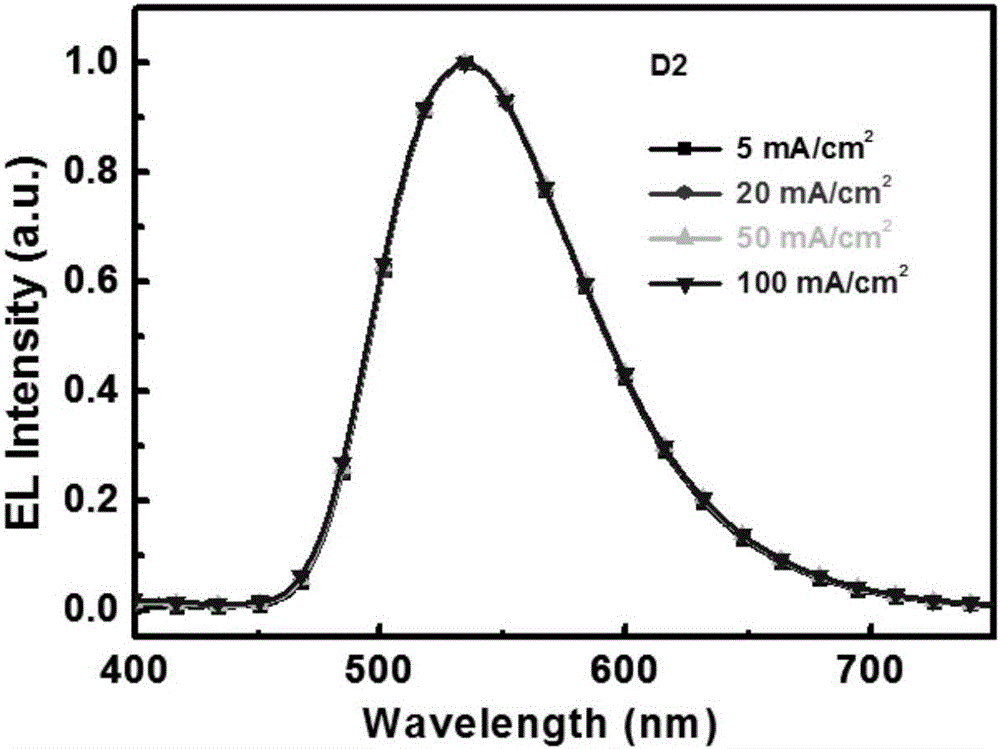
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 2,9-di-N-phenoxazinyl-1,10-phenanthroline
[0035]
[0036] 2,9-di-N-phenoxazinyl-1,10-phenanthroline
[0037] Add 1.3 g of phenoxazine, 240 mg of 70% oil-dispersed sodium hydride and 20 ml of tetrahydrofuran into a 50 ml single-necked round-bottom flask, reflux for 30 minutes under argon protection, and then add 0.75 g of 2,9-dichloro-1, 10 phenanthroline, then refluxed at 60 degrees Celsius for 24 hours, cooled to room temperature, quenched with saturated brine, extracted with dichloromethane, dried the organic phase with anhydrous sodium sulfate, filtered, and spin-dried. The product was passed through the column with methanol:dichloromethane volume ratio 1:20 to obtain 1.35 g of the product. Yellow-green solid, yield 83%. 1H NMR (CDCl 3 ,400MHz): δ[ppm]8.17(d,J=8Hz,2H),7.77(d,J=8Hz,4H),7.71(s,2H),7.65(d,J=8Hz,2H),6.96( d,J=4Hz,8H),6.88-6.84(m,4H).MS(EI):m / z 542.3[M] + .
Embodiment 2
[0038] Example 2: Preparation of 4,7-di-N-phenoxazinyl-1,10-phenanthroline
[0039]
[0040] 4,7-di-N-phenoxazinyl-1,10-phenanthroline
[0041] Add 1.30 g of phenoxazine, 240 mg of 70% oil-dispersed sodium hydride and 20 ml of tetrahydrofuran into a 50 ml single-necked round-bottomed flask, and add 0.75 g of 4,7-dichloro-1 after reflux for 30 minutes under argon protection. 10 phenanthroline, then refluxed at 60 degrees Celsius for 24 hours, cooled to room temperature, quenched with saturated brine, extracted with dichloromethane, dried the organic phase with anhydrous sodium sulfate, filtered, and spin-dried. The product was passed through the column at a volume ratio of methanol:dichloromethane of 1:30 to obtain 1.40 g of the product. Pale yellow solid, yield 86%. 1 H NMR (CDCl 3 ,400MHz): δ[ppm]9.47(d,J=8Hz,2H),8.07(s,2H),7.77(d,J=4Hz,2H),6.77(d,J=8Hz,4H),6.71- 6.67(m,4H),6.54-6.50(m,4H),5.73(d,J=8Hz,4H).MS(EI):m / z 542.4[M] + .Example 3: Preparation of 4,7-di-N-phen...
Embodiment 3
[0041] Add 1.30 g of phenoxazine, 240 mg of 70% oil-dispersed sodium hydride and 20 ml of tetrahydrofuran into a 50 ml single-necked round-bottomed flask, and add 0.75 g of 4,7-dichloro-1 after reflux for 30 minutes under argon protection. 10 phenanthroline, then refluxed at 60 degrees Celsius for 24 hours, cooled to room temperature, quenched with saturated brine, extracted with dichloromethane, dried the organic phase with anhydrous sodium sulfate, filtered, and spin-dried. The product was passed through the column at a volume ratio of methanol:dichloromethane of 1:30 to obtain 1.40 g of the product. Pale yellow solid, yield 86%. 1 H NMR (CDCl 3 ,400MHz): δ[ppm]9.47(d,J=8Hz,2H),8.07(s,2H),7.77(d,J=4Hz,2H),6.77(d,J=8Hz,4H),6.71- 6.67(m,4H),6.54-6.50(m,4H),5.73(d,J=8Hz,4H).MS(EI):m / z 542.4[M] + .Example 3: Preparation of 4,7-di-N-phenothiazinyl-1,10-phenanthroline
[0042]
[0043] 4,7-di-N-phenothiazinyl-1,10-phenanthroline
[0044] Add 1.41 g of phenothiazine, 240 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com