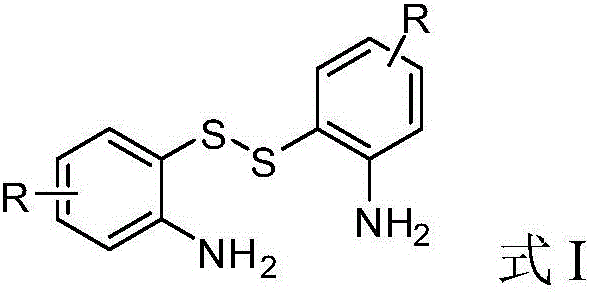
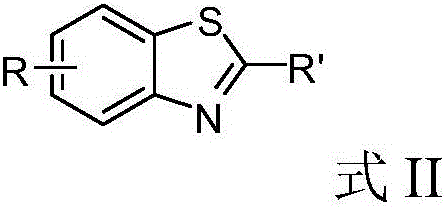
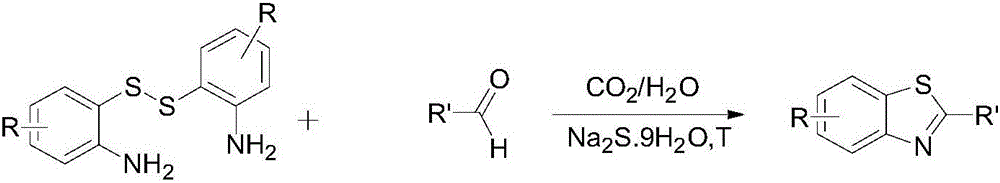
Method for realizing green synthesis of 2-substituted benzothiazole derivatives
A technology for benzothiazoles, green synthesis, applied in the direction of organic chemistry and the like, can solve the problems of unstable o-aminothiophenol, high cost of raw material preparation, long synthesis steps, etc., and achieves short steps, high yield and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: take benzaldehyde as raw material to synthesize 2-phenylbenzothiazole
[0030] (1) Synthesis of 2-phenylbenzothiazole
[0031] a: Disulfide: Aldehyde: Na 2 S·9H 2 O=1:2:0.2
[0032] In the autoclave, add 0.40mmol of 2,2'-dithiodiphenylamine, 0.80mmol of benzaldehyde and 0.08mmol of Na 2 S·9H 2 O, put the magneton, then add 2 mL of water as the reaction solvent, heat it to 50 °C, and then fill with 2 MPa of carbon dioxide gas. Then continue to heat up to 80°C and stir for 24h. LC detection finds that the reaction of the disulfide raw materials is complete, cool to room temperature, and use a rotary evaporator to remove the solvent under reduced pressure to obtain a crude product. Using petroleum ether and ethyl acetate as eluents, the crude product was eluted with a gradient, and separated by column chromatography (200-300 mesh silica gel) to obtain 121.5 mg of 2-phenylbenzothiazole as a white powder with a purity of more than 99%. , the isolated yiel...
Embodiment 2
[0044] Example 2: Synthesis of 2-(4-chlorophenyl) benzothiazole with p-chlorobenzaldehyde as raw material
[0045] (1) Synthesis of 2-(4-chlorophenyl)benzothiazole
[0046] In the autoclave, add 0.40mmol of 2,2'-dithiodiphenylamine, 0.80mmol of p-chlorobenzaldehyde and 0.2mmol of Na 2 S·9H 2 O, put the magneton, then add 2 mL of water as the reaction solvent, heat it to 50 °C, and then fill with 2 MPa of carbon dioxide gas. Then continue to heat up to 80°C and stir for 24h. LC detection finds that the reaction of the disulfide raw materials is complete, cool to room temperature, and use a rotary evaporator to remove the solvent under reduced pressure to obtain a crude product. Using petroleum ether and ethyl acetate as eluents, the crude product was eluted with a gradient and separated by column chromatography (200-300 mesh silica gel) to obtain 2-(4-chlorophenyl) as a white powder with a purity greater than 99%. Benzothiazole 139.2 mg, isolated yield 71%, mp 112-113°C.
...
Embodiment 3
[0052] Embodiment 3: take p-bromobenzaldehyde as raw material to synthesize 2-(4-bromophenyl) benzothiazole
[0053] (1) Synthesis of 2-(4-bromophenyl)benzothiazole
[0054] In the autoclave, add 0.40mmol of 2,2'-dithiodiphenylamine, 0.80mmol of p-bromobenzaldehyde and 0.2mmol of Na 2 S·9H 2 O, put the magneton, then add 2 mL of water as the reaction solvent, heat it to 50 °C, and then fill with 2 MPa of carbon dioxide gas. Then continue to heat up to 80°C and stir for 24h. LC detection finds that the reaction of the disulfide raw materials is complete, cool to room temperature, and use a rotary evaporator to remove the solvent under reduced pressure to obtain a crude product. Using petroleum ether and ethyl acetate as eluents, the crude product was eluted with a gradient and separated by column chromatography (200-300 mesh silica gel) to obtain 2-(4-bromophenyl) as a white powder with a purity greater than 99%. Benzothiazole 187.2 mg, isolated yield 81%, mp 126-128°C.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com