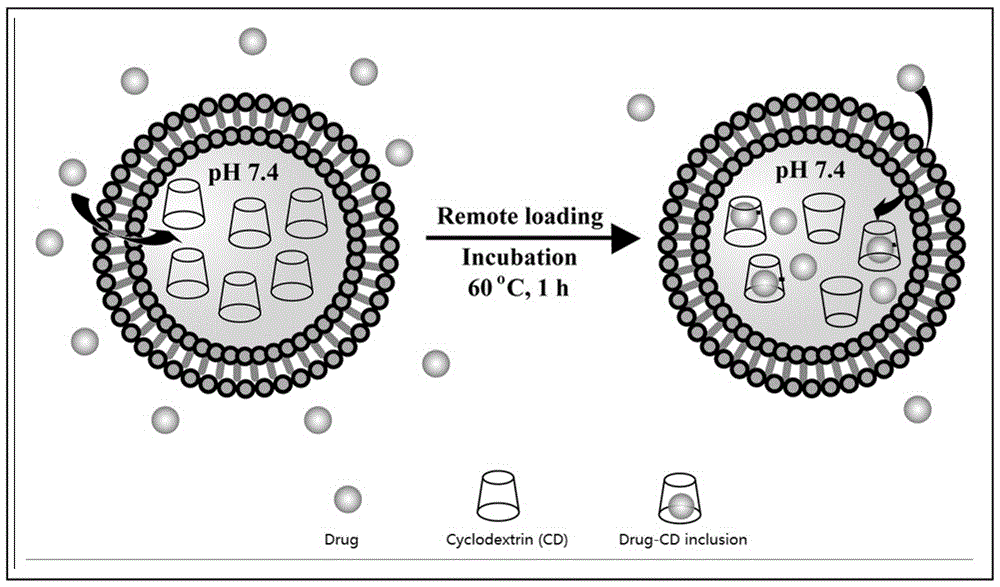
Lipidosome medicine carrying method
A liposome and blank liposome technology, applied in the field of biomedicine, can solve the problems of phospholipid decomposition, deterioration, lack of drug-carrying methods, etc., and achieve the effects of high stability and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 300 mM α-cyclodextrin (α-CD) was used as the aqueous phase, and phosphatidylcholine and phosphatidylserine (SPC / SPS = 10:1, mole ratio) were used as membrane materials, and the package was prepared by emulsification-lyophilization method. Seal α-CD liposomes, remove free α-CD by centrifugation, and obtain blank liposomes encapsulating cyclodextrin (cyclodextrin: liposome membrane material = 1:5, mass ratio); then add baicalein, Drug / phospholipid (1:20, w / w), incubate at 40 °C for 1 hour to complete active drug loading; observe the liposome as a spherical nanoparticle by electron microscope, the average particle size detected by DLS is 220 nm, PDI = 0.36, ζ = At -30 mV, the encapsulation efficiencies detected by HLPC were 92%, 95%, and 99%, respectively. (SPC, soybean phosphatidylcholine; SPS, soybean phosphatidylserine; DLS, dynamic light scattering; PDI, polydispersity index).
Embodiment 2
[0026] Preparation of liposomes loaded with amphoteric drugs by CRL method
[0027] Using 100 mM PEG2000-hydroxypropyl-β-cyclodextrin (PEG-HP-β-CD) as the aqueous phase, phospholipids and cholesterol (DPPC / CHO = 1:1, mole ratio) as the membrane material, the package was prepared by the reverse evaporation method. Encapsulate HP-β-CD liposomes, remove free PEG-HP-β-CD by column chromatography, and obtain blank liposomes encapsulating cyclodextrin (cyclodextrin: liposome membrane material = 1:10, mass Ratio); then add Folate-PEG2000-DSPE (FPD) and topotecan (FPD / DPPC = 1:20, moleratio, drug / DPPC = 1:20, w / w), incubate at 45 °C for 1 hour, and complete the lipolysis Folic acid modification and active drug loading on the surface of plastids; the liposomes are spherical nanoparticles observed by electron microscopy, the average particle size detected by DLS is 300 nm, and the distribution is relatively uniform (PDI = 0.23), ζ = -5mV, (high performance liquid phase) HLPC The detect...
Embodiment 3
[0029] Preparation of oligopeptide-loaded vesicles by CRL method
[0030] 300 mM hydroxypropyl-β-cyclodextrin (HP-β-CD) and 5% sucrose were used as the aqueous phase, and SPAN 80 (SPAN80) and DOTAP (SPAN / DOTAP = 20:1, mole ratio) were used as membrane materials. Prepare positively charged vesicles encapsulating HP-β-CD by dispersion-extrusion method, remove free HP-β-CD by dialysis, and obtain blank liposomes encapsulating cyclodextrin (cyclodextrin: liposome membrane material = 1:15, mass ratio); then add glutathione (Glutathione / SPAN = 1:30, w / w) and incubate at 40 °C for 0.5 h to complete active drug loading; observe the liposomes as spherical nanoparticles by electron microscope , the average particle size detected by DLS is 200 nm, the distribution is uniform (PDI=0.23), ζ = 35mV, and the encapsulation efficiency detected by HLPC is 75%. The drug-loaded vesicles were further freeze-dried, and the freeze-dried product was stored at 4 °C for 6 months. After hydration, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com