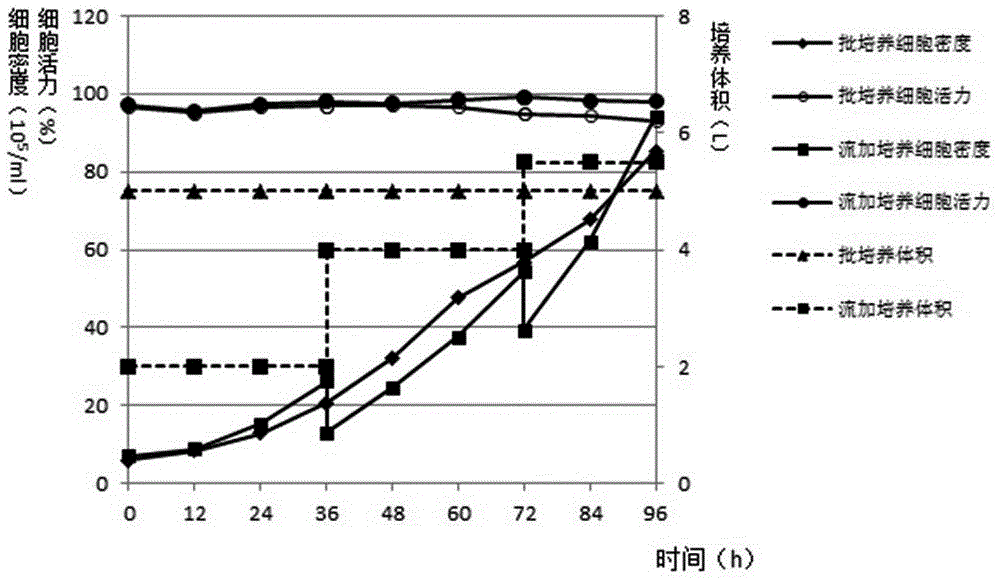
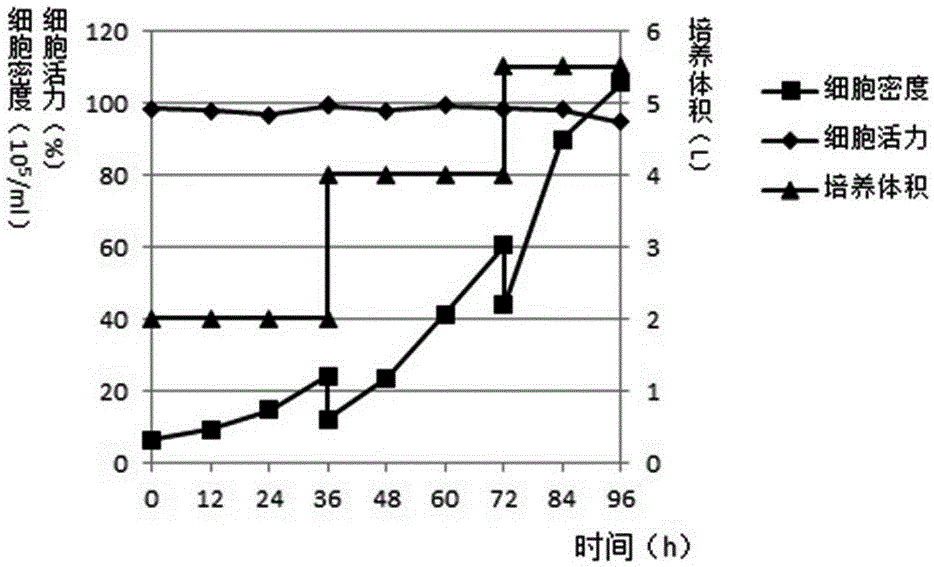
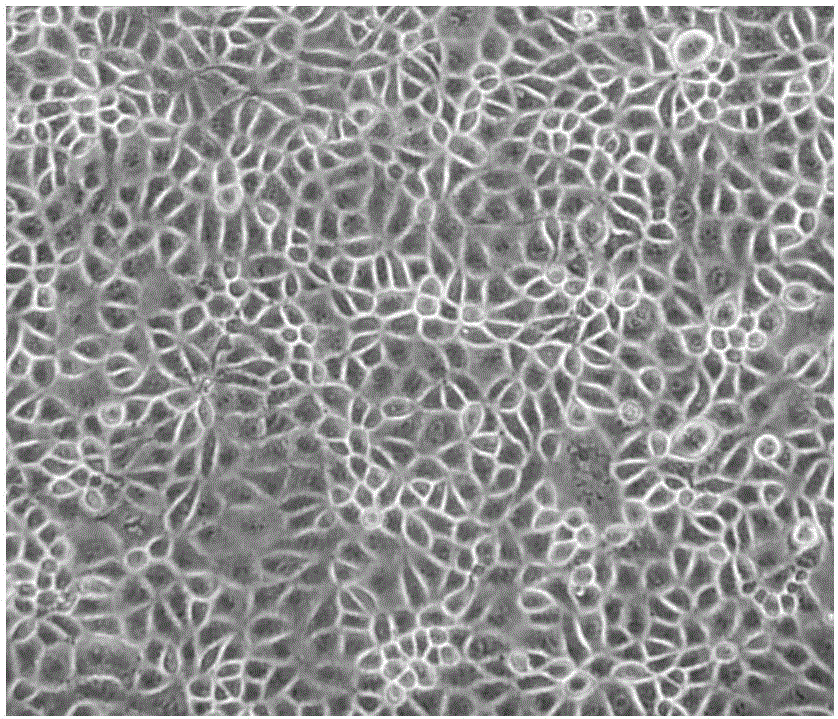
Domestication method of full suspension culture type Marc-145 cell line
A full-suspension, cell-line technology, applied in the methods of using microorganisms, biochemical equipment and methods, embryonic cells, etc., can solve the problems of unsolved technical problems, high prices, and expensive manual operations, and achieve good social benefits, The effect of saving space and manpower
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Materials:
[0043] 1) Cell line: Marc-145 rhesus monkey embryonic kidney cells, provided by Animal Cell Engineering Technology Research Center of Gansu Province;
[0044] 2) DMEM medium, Gibco, product number: 02-5062EJ;
[0045] 3) DMEM / F12 culture medium, Gibco, product number: 12500-096;
[0046] 4) Serum-free medium No. 1, Lanzhou Bailing Biotechnology Co., Ltd., item number: BFLM401.05;
[0047] 5) Serum-free medium No. 2, Lanzhou Bailing Biotechnology Co., Ltd., item number: BFLM101.05;
[0048] 6) Low serum medium, Lanzhou Bailing Biotechnology Co., Ltd., item number: BLLM101.05;
[0049] 7) Newborn bovine serum, Lanzhou Minhai Biological Engineering Co., Ltd., batch number: 20150716;
[0050] 8) DMSO (dimethyl sulfoxide), Thermo Fisher Scientific Corporation, item number: 20688 8);
[0051] 9) Trypsin, Gibco, product number: 27250, prepared as 0.25% trypsin solution (EDTA-Na 0.3g / L).
[0052] 2. The equipment and utensils used
[0053] 1) Bioreactor, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com