Asymmetric cationic Gemini surfactant containing hydroxyl group in coupling link
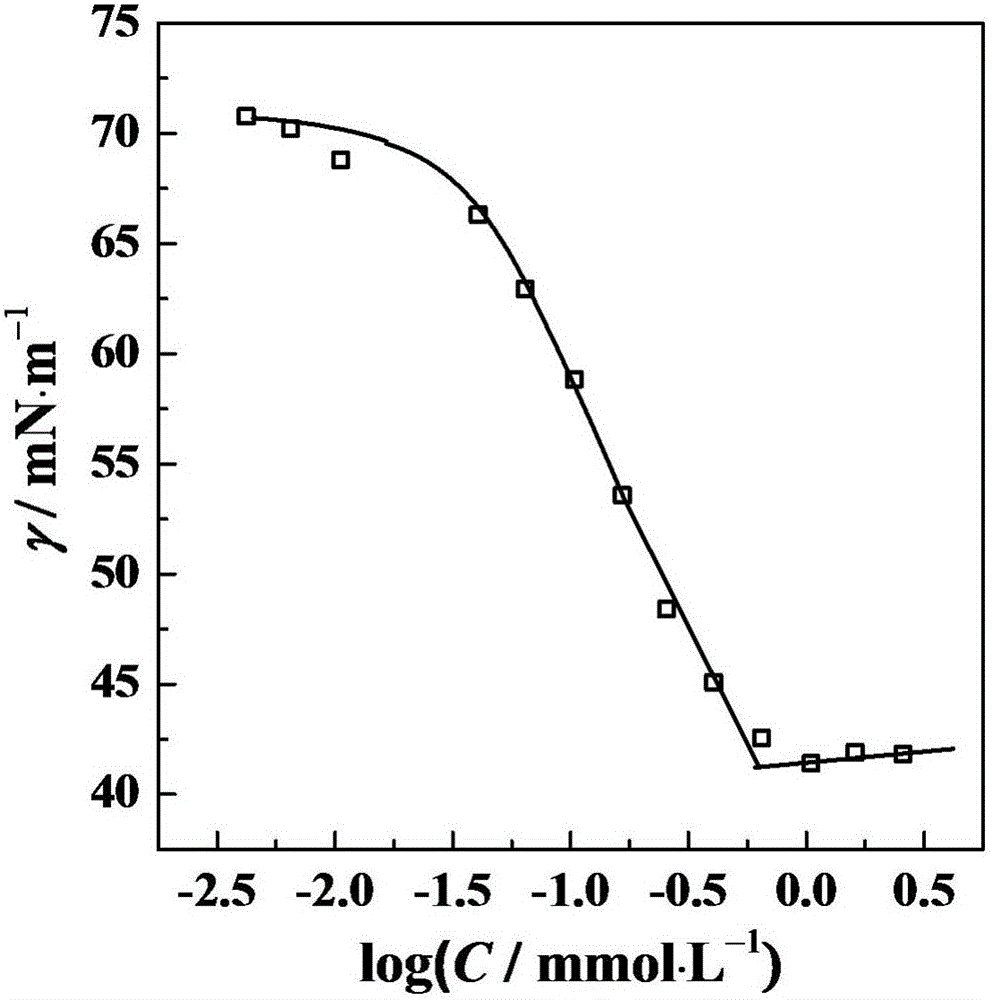
A technology of surfactants and hydroxyl groups, which is applied in the field of asymmetric cationic Gemini surfactants, can solve the problems of limiting the application range of Gemini surfactants, and achieve the effect of enhanced aggregation ability, excellent performance and high surface activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
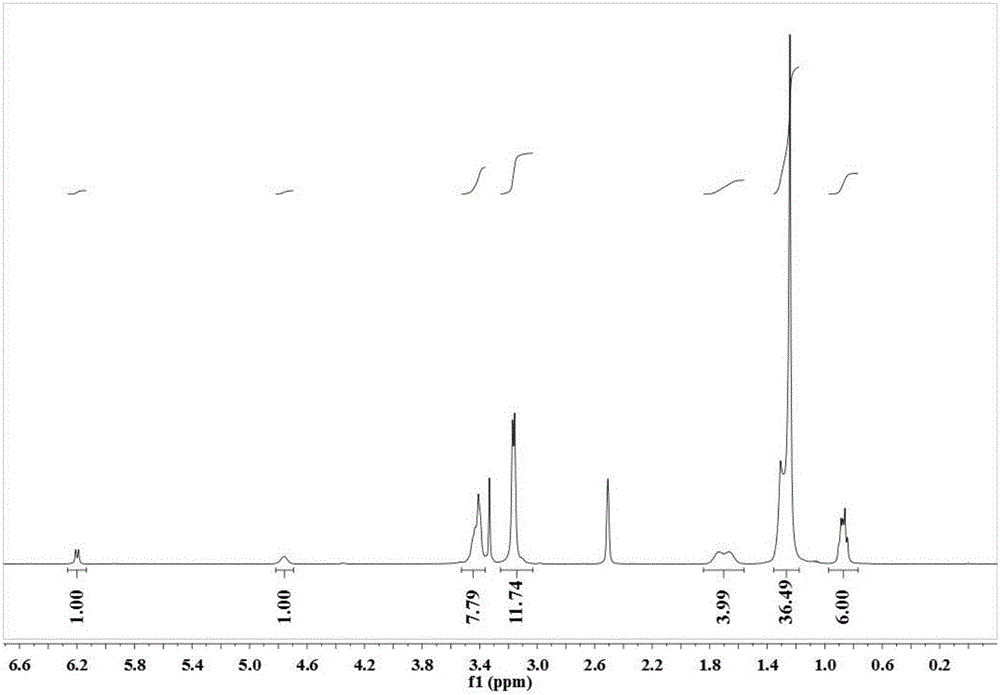
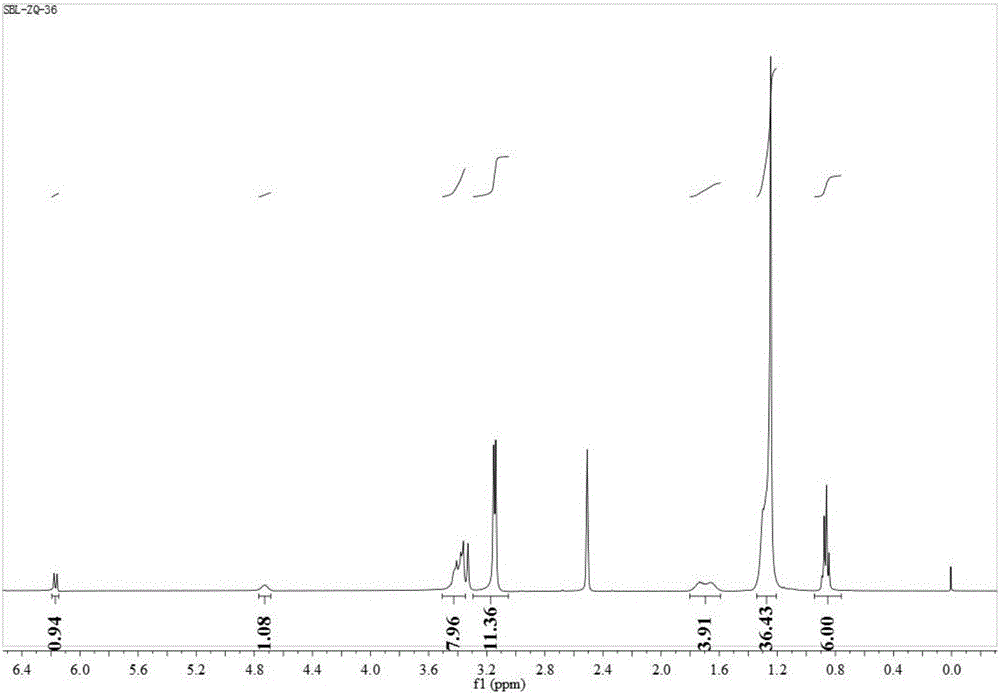
[0028] Embodiment 1: 6-3(OH)-18 synthetic route
[0029] The asymmetrical cationic Gemini surfactant 6-3(OH)-18 containing a hydroxyl group in the connecting chain has the following structural formula:
[0030]
[0031] The synthetic route is as follows:
[0032] Synthetic route of 6-3(OH)-18
[0033]
[0034] Using N,N-dimethyl n-hexylamine and 1,3-dibromoisopropanol as raw materials, synthesize the unilateral substitution product product 2-hydroxy-3-bromohexane ammonium bromide; 2-hydroxy-3-bromohexyl Alkyl ammonium bromide reacted with N,N-dimethyl octadecyl amine to synthesize a new type of cationic Gemini surfactant with hydroxyl group in the asymmetric linkage chain.
Embodiment 2
[0035] Embodiment 2: the synthesis of intermediate product
[0036] Synthesis of 2-Hydroxy-3-Bromohexane Ammonium Bromide
[0037] Mix N,N-dimethyl-n-hexylamine and 1,3-dibromoisopropanol at a molar ratio of 1:4, add absolute ethanol, stir at 70°C for 12 hours, and cool down. Ethanol was removed by rotary evaporation. Add ether for extraction (3 times), let stand for 10 min, and collect the viscous liquid in the lower layer. After filtration, the filtrate was rotary evaporated to remove diethyl ether to obtain the product. Yield: 41.8%.
Embodiment 3
[0038] Embodiment 3: quaternization reaction synthesis product
[0039] Synthesis of 6-3(OH)-18
[0040] 2-Hydroxy-3-bromohexylammonium bromide and N,N-dimethyloctadecylamine were mixed in a molar ratio of 1:1 in the reactor, and reacted at 80°C for 24 hours. After the reaction was cooled, ethanol was removed by rotary evaporation. The filtrate was obtained by recrystallization with ethanol / ethyl acetate mixed solvent, and the filtrate was recrystallized three times with ethanol / anhydrous diethyl ether mixed solvent to obtain the final product, which was a white solid. Yield: 21.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com