Transition metal nanoparticle catalyst with dual confinement structure as well as application thereof to catalysis of selective hydrogenation reaction of dimethyl terephthalate
A technology of dimethyl phthalate and transition metals, which is applied in the field of transition metal nanoparticle catalysts, can solve the problems of reduced Ni metal reduction, large active metal nanoparticles, and difficult oxide reduction, so as to prevent sintering and Loss of transition metals, strong interaction, and outstanding reaction stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
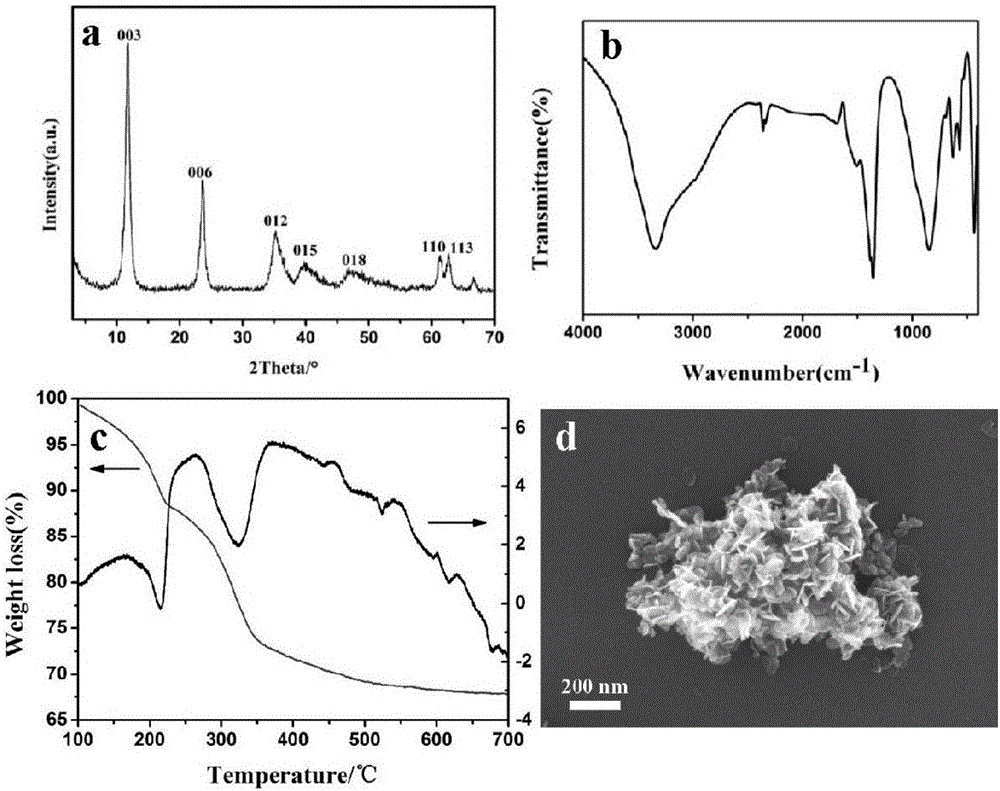
[0021] A. Add 19.2g of Ni(NO 3 ) 2 ·6H 2 O, 12.4g of Al(NO 3 ) 2 9H 2 O was added to 150mL deionized water, and ultrasonically dissolved to obtain a mixed salt solution; 6.3g of sodium hydroxide and 7.0g of sodium carbonate were added to 150mL of deionized water, and ultrasonically dissolved to obtain a mixed alkali solution; 150mL of deionized water was put into 500mL In the three-necked flask, then gradually drop the mixed alkali solution to pH 10, then drop the mixed salt solution simultaneously to keep the pH at 10; Crystallize at 130°C for 24h, filter, wash with deionized water and filter to pH 7, and finally dry at 70°C for 24h to obtain a highly dispersed hydrotalcite precursor, denoted as Ni 2 Al-LDHs (XRD, FT-IR, TG-DTA spectra and SEM images see figure 1 );
[0022] B. The highly dispersed hydrotalcite precursor Ni prepared in step A 2 Al-LDHs is placed in a high-temperature atmosphere furnace, and H with a purity of 99.999% is introduced 2 , the flow rate i...
Embodiment 2
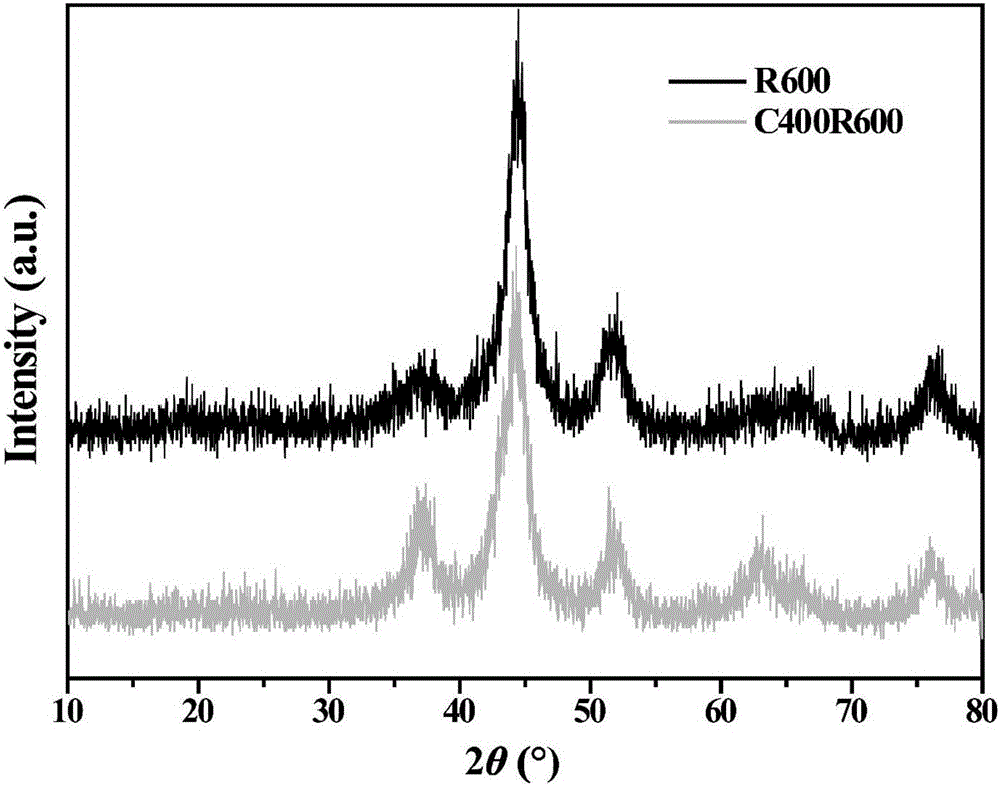
[0026]Place the hydrotalcite precursor prepared in step A in Example 1 in a muffle furnace, air roast, raise the temperature in the furnace to 400°C, the heating rate is 5°C / min, keep it for 5h, and then naturally cool to room temperature; after taking it out Then place it in a high-temperature atmosphere furnace and feed H2 with a purity of 99.999%. 2 , the flow rate is 60mL / min, the temperature in the furnace is raised to 400°C and 600°C respectively, the heating rate is 5°C / min, keep for 5h, and then naturally cooled to room temperature, the transition metal nanoparticle catalysts with double confinement structure are obtained respectively denoted as Ni / NiAlO x / AlO x (C400R400) and Ni / NiAlO x / AlO x (C400R600) sample (XRD figure see figure 2 ).
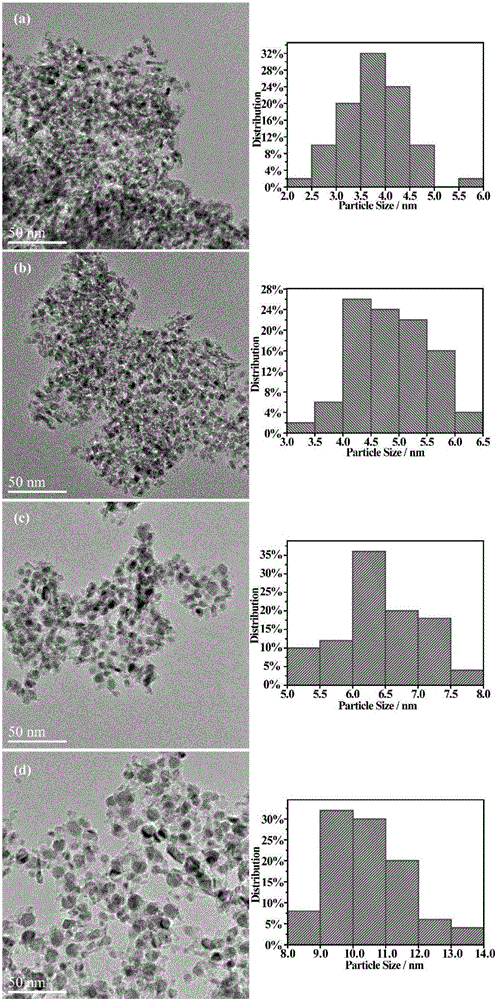
[0027] The transition metal nanoparticle catalyst with a double confinement structure prepared above has a composition structure: nickel nanoparticles are confined in an aluminum-doped nickel oxide shell, and the outermost p...
Embodiment 3
[0032] A. Add 19.2g of Co(NO 3 ) 2 ·6H 2 O, 12.4g of Al(NO 3 ) 2 9H 2 O was added to 150mL deionized water, and ultrasonically dissolved to obtain a mixed salt solution; 6.3g of sodium hydroxide and 7.0g of sodium carbonate were added to 150mL of deionized water, and ultrasonically dissolved to obtain a mixed alkali solution; 150mL of deionized water was put into 500mL In the three-necked flask, then gradually drop the mixed alkali solution to pH 10, then drop the mixed salt solution simultaneously to keep the pH at 10; Crystallized at 130 °C for 24 h, filtered, washed with deionized water and filtered to pH 7, and finally dried at 70 °C for 24 h to obtain a highly dispersed hydrotalcite precursor, denoted as Co 2 Al-LDHs;
[0033] B. The highly dispersed hydrotalcite precursor Co prepared in step A 2 Al-LDHs is placed in a high-temperature atmosphere furnace, and H with a purity of 99.999% is introduced 2 , the flow rate is 60mL / min, the temperature in the furnace is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com