Synthesis method for 4-hydroxyisoleucine
A technology of hydroxyisoleucine and a synthesis method is applied in the field of compound biotechnology production, and can solve the problems of difficult preparation, many steps and high preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
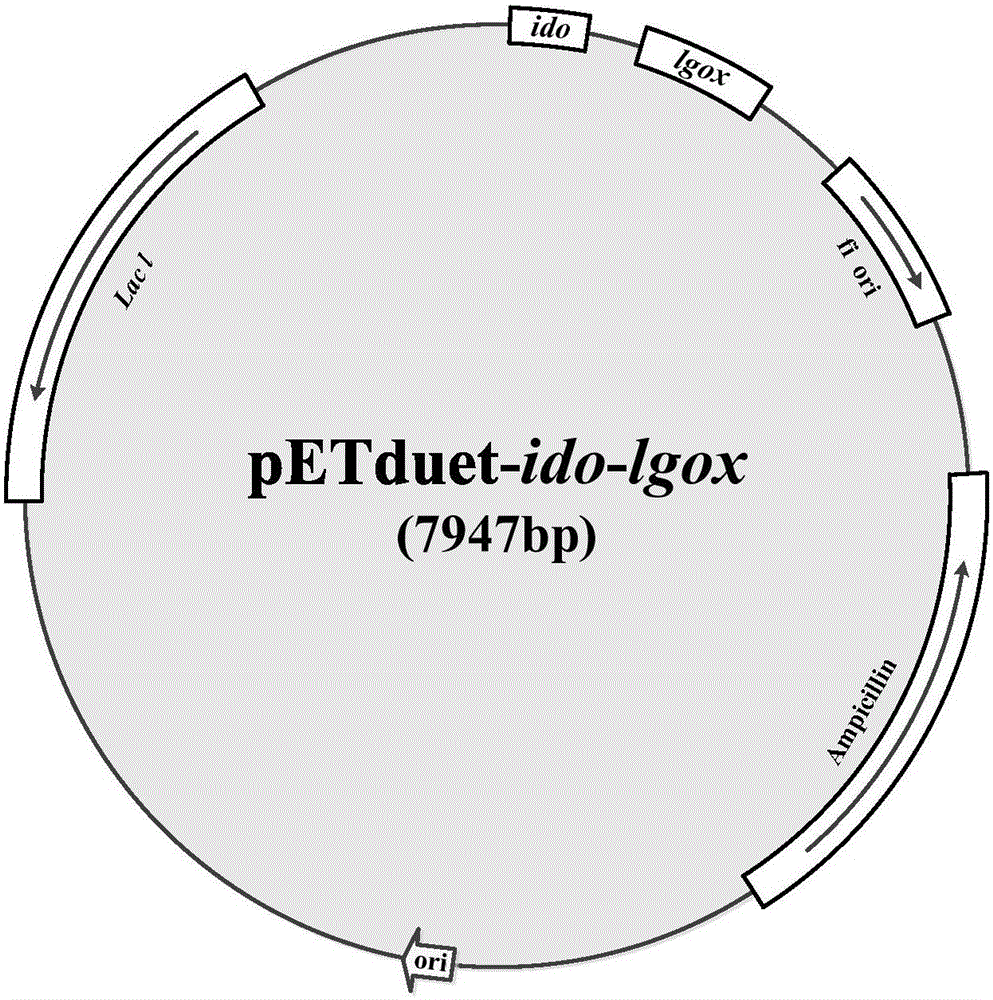
[0025] Construct strain E.coli BL21(DE3) / pETduet-ido-lgox and express L-isoleucine-4-hydroxylase and L-glutamic acid oxidase, using substrates L-glutamic acid and L-iso Enzymatic Synthesis of 4-Hydroxyisoleucine from Leucine
[0026] (1) Obtain ido and lgox nucleotide sequences
[0027] According to the nucleotide sequences of ido and lgox shown in Sequence Listing 1 and 2, the nucleotide sequences shown in Sequence Listing 3 and 4 were obtained by performing codon optimization on E.coli BL21(DE3) acid sequence. ido and lgox are exogenous genes, in order to increase their expression in the host E.coli BL21(DE3), it is necessary to optimize their codons to the most frequently used sequences in the host, wherein,
[0028] ido optimized nucleotide sequence for E.coli BL21(DE3), primers:
[0029] Upstream primer: 5'TAT GGATCC ATGAAAATGAGCGGTTTTAGCA 3'
[0030] Downstream primer: 5'CCC AAGCTT TTATTTGGTTTCTTTATAGCTAAAGGTC 3'
[0031] lgox optimized nucleotide sequence for E...
Embodiment 2
[0045] Embodiment 2 Enzyme Catalytic Synthesis of 4-Hydroxyisoleucine
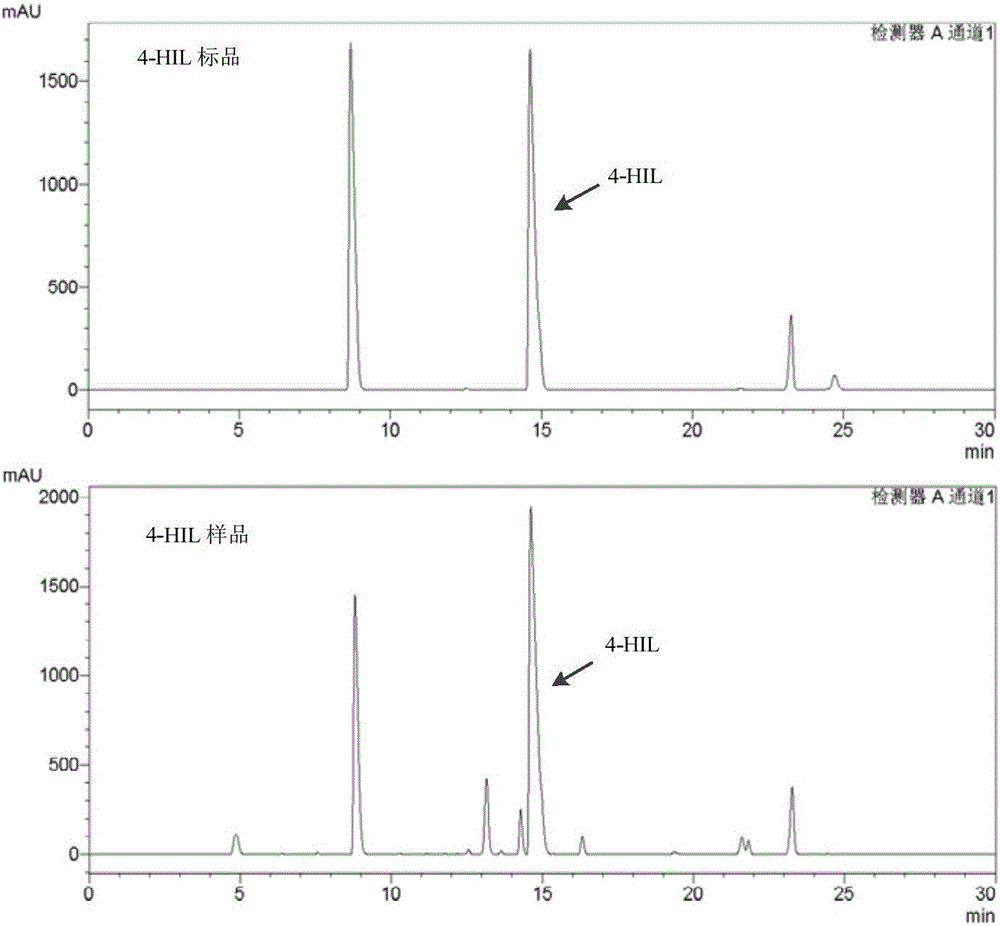
[0046] Add the crude enzyme obtained in Example 1(4) into the prepared reaction buffer, the final concentration is 20g / L; catalyze the reaction at 30°C and 150rpm in a water bath shaker for 4h, centrifuge and take the supernatant to obtain the product 4-Hydroxyisoleucine. As detected by HPLC, after 4 hours of catalytic reaction, the conversion rates of L-glutamic acid and L-isoleucine were 91.2% and 93.5%, respectively.
[0047] The above reaction buffer preparation method: L-glutamic acid 20g / L, L-isoleucine 15g / L, Fe 2+ 20mmol / L, Vc 5mmol / L, 0.1mol / L sodium carbonate-sodium bicarbonate buffer solution (the configuration method is to dissolve 21.2g sodium carbonate and 67.2g sodium bicarbonate in water and dilute to 1L), pH 9.1.
Embodiment 3
[0048] Embodiment 3 enzymatic method synthesizes 4-hydroxyisoleucine
[0049] Add the crude enzyme obtained in Example 1(4) into the prepared reaction buffer, the final concentration is 30g / L; catalyze the reaction at 32°C and 150rpm in a water-bath shaker for 4h, centrifuge to take the supernatant, and obtain the product 4-Hydroxyisoleucine. As detected by HPLC, after 4 hours of catalytic reaction, the conversion rates of L-glutamic acid and L-isoleucine were 97.01% and 98.45%, respectively.
[0050] The above reaction buffer preparation method: L-glutamic acid 20g / L, L-isoleucine 15g / L, Fe 2+ 20mmol / L, Vc 5mmol / L, Hepes 50mmol / L, pH 7.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com