Liquid preparation, and preparation method and application thereof
A liquid preparation and aqueous solution technology, applied in the field of medicine, can solve the problems of weak liver and kidney function, increase the burden on children's liver and kidney, etc., and achieve the effect of strong antibacterial ability and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1 Sample 1 # ~Sample 6 # preparation of
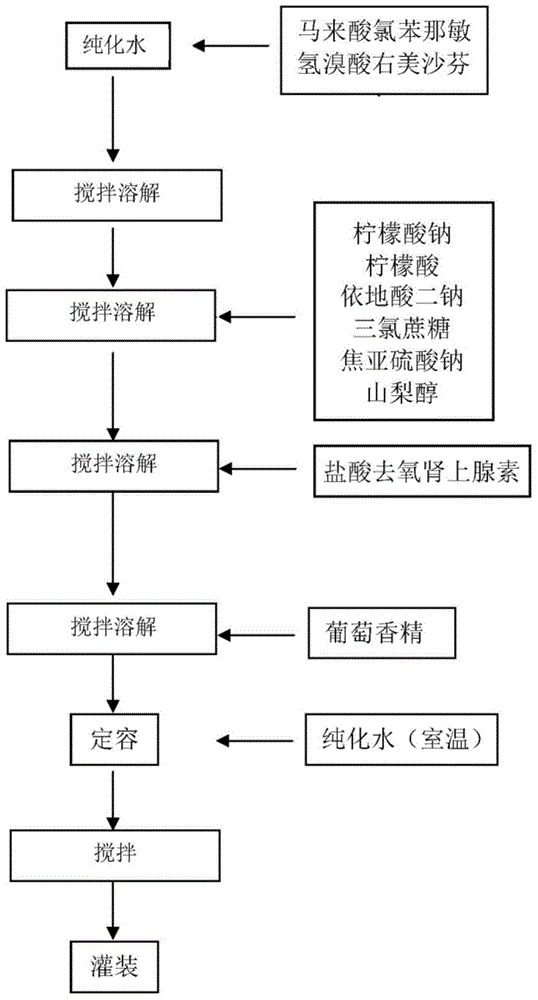
[0108] The sample preparation steps are:
[0109] a) dissolving chlorpheniramine maleate and dextromethorphan hydrobromide in water to obtain aqueous solution I;
[0110] b) sequentially adding sodium citrate, citric acid, disodium edetate, sucralose, sodium metabisulfite and sorbitol to the aqueous solution obtained in step a), stirring and dissolving to obtain aqueous solution II;
[0111] c) adding phenylephrine hydrochloride to the aqueous solution II obtained in step b) and stirring to dissolve to obtain aqueous solution III;
[0112] d) Add grape essence to the aqueous solution III obtained in step c), stir evenly, and dilute with purified water to obtain the liquid preparation.
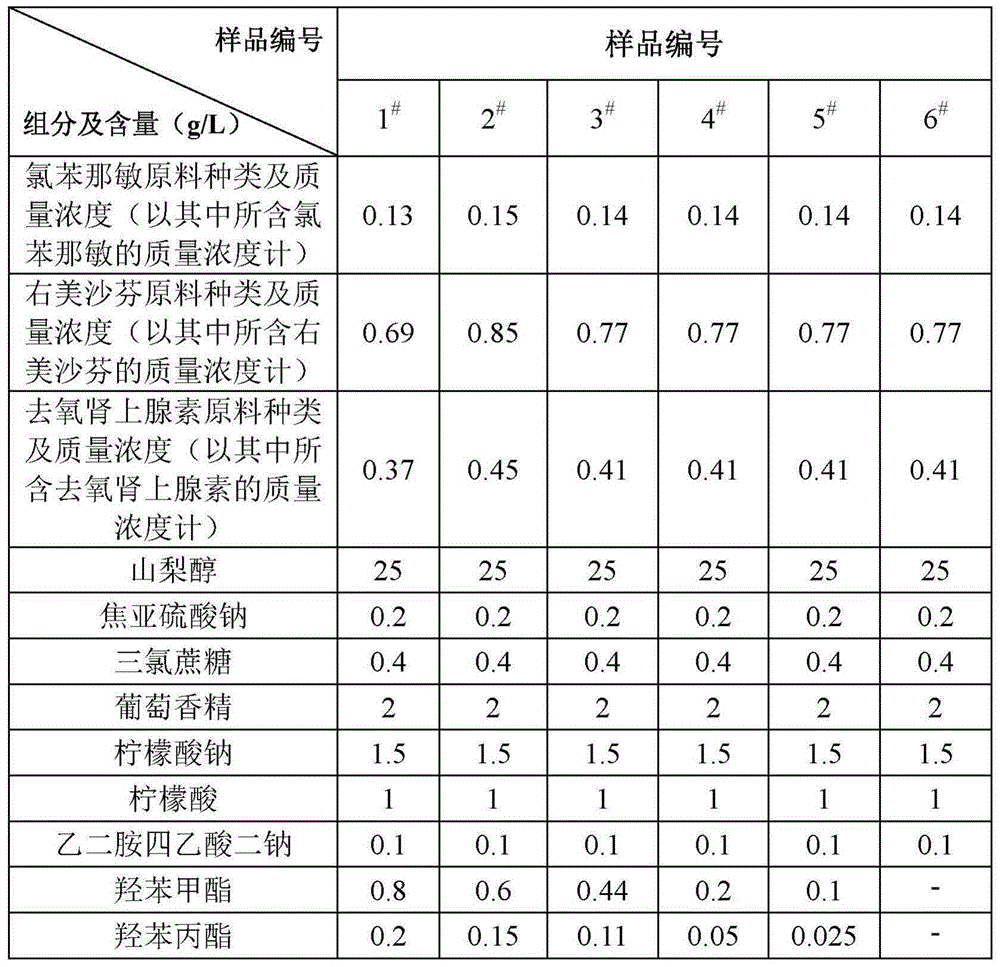
[0113] The relationship between the type and mass concentration of each component in the liquid preparation and the sample number is shown in Table 1.
[0114] Table 1
[0115]
Embodiment 2
[0116] Example 2 Sample 1 # ~Sample 6 # Determination of antibacterial ability
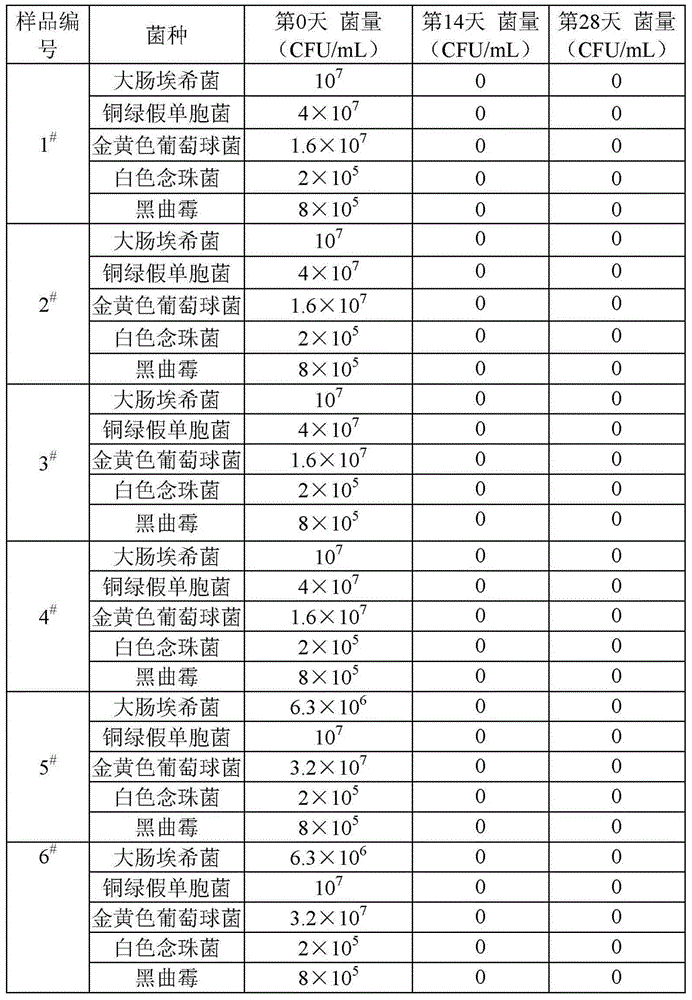
[0117] Get sample 1 obtained in embodiment 1 respectively # ~Sample 6 #Each of five samples was inoculated with Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, and Aspergillus niger, and cultured in an incubator. On the 28th day, samples were taken and the viable bacteria content in the samples was determined. The initial viable bacteria content and test results are shown in Table 2.
[0118] Table 2 Sample antibacterial ability test
[0119]
[0120] As can be seen from the data in Table 2, the liquid preparation provided by the technical solution of the present application still has a strong antibacterial effect and can achieve a complete antibacterial effect when the formula does not contain organic solvents and preservatives. .
Embodiment 3
[0121] Embodiment 3 accelerated test
[0122] According to the experimental procedure and formula in embodiment 1, repeat preparation sample 6 respectively # , to investigate the repeatability of the samples obtained during the preparation process. Take samples separately 6 # Samples from three different batches are recorded as sample 6 according to different batches # -n where n represents the batch number. For example sample 6 # -1 is the first batch of samples 6 # , sample 6 # -2 is the second batch of sample 6 # , sample 6 # -3 is the third batch of sample 6 # .
[0123] Respectively for the above sample 6 # -1~sample 6 # -3. A total of 3 batches of samples were used for accelerated experiments.
[0124] The test conditions are: stored at a temperature of 40±2°C and a relative humidity of 75±5%, at the 0th month, 1st month, 2nd month, 3rd month, and 6th month of storage respectively Sampling and analyzing the content of active ingredients, related substances a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com