High-purity lubiprostone compound and preparation method thereof
A technology for lubiprostone and compound is applied in the field of preparation of high-purity lubiprostone compound, can solve the problems of complicated operation, high cost, low yield and the like, and achieves the effects of high total reaction yield and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
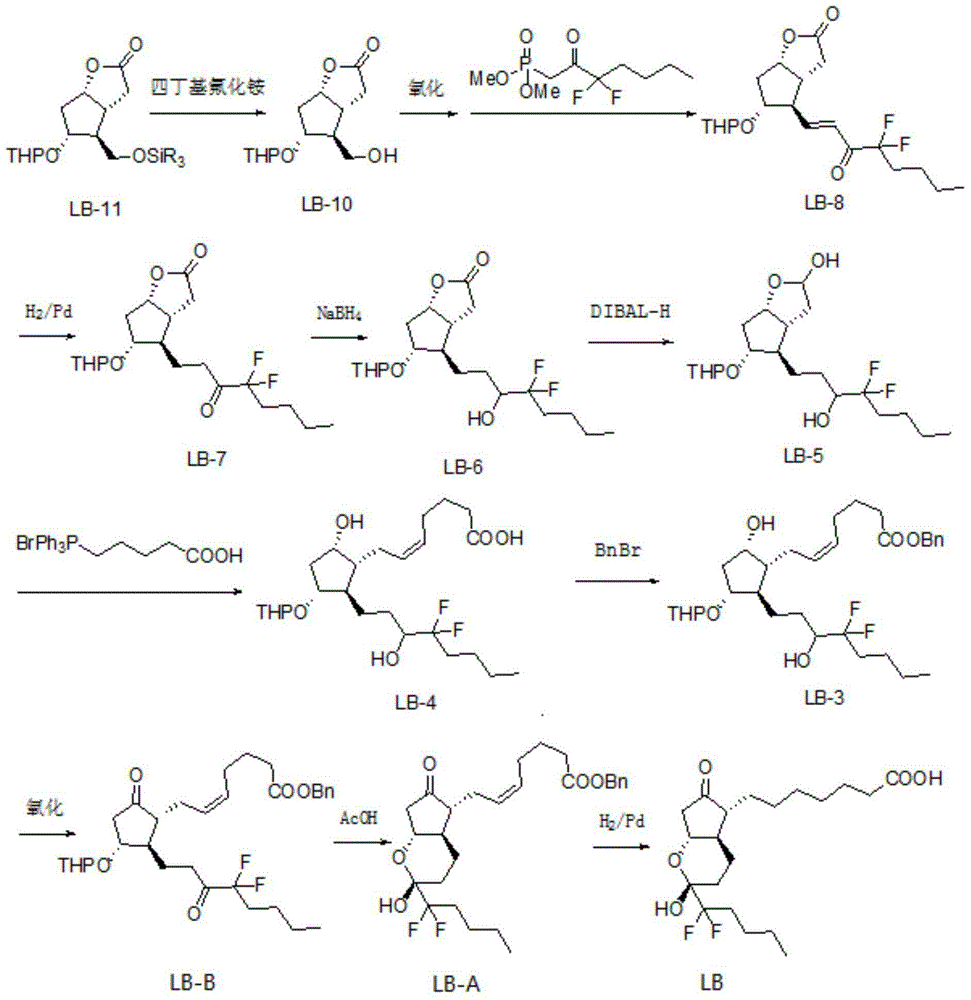
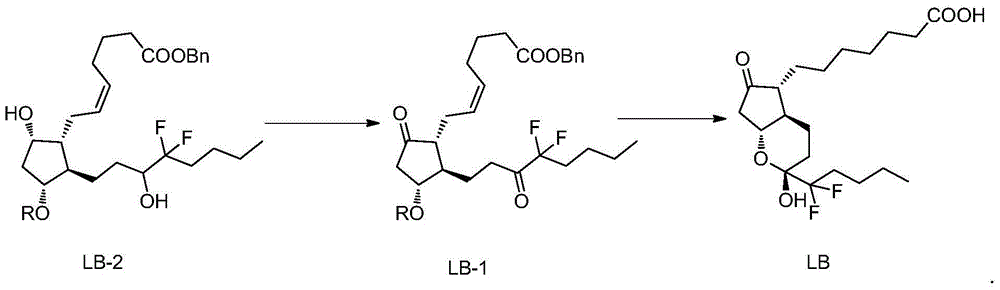
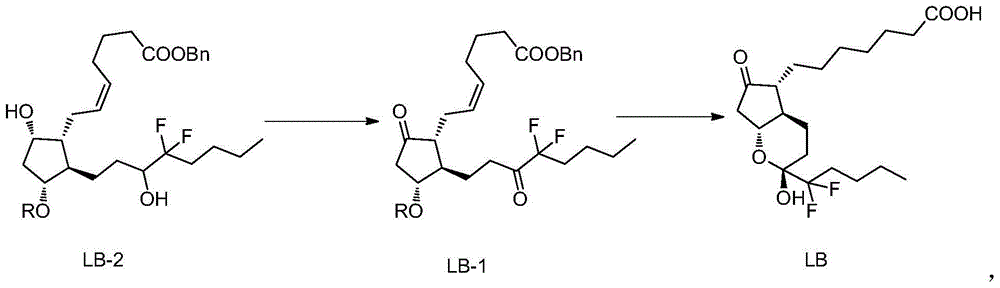
[0029] Example 1: (Z)-7-[(1R,2R,3R)-2-(4,4-difluoro-3-oxooctyl)-5-oxo-3-(benzyl-2- Preparation of benzyl oxo)-cyclopentyl]-5-heptenoate (LB-1)
[0030] Mix Dess-Martin oxidant (80g) with dichloromethane (400mL), cool in an ice-water bath, control the internal temperature at 0-10°C, add (Z)-7-[(1R,2R,3R,5S)-2-( Benzyl 4,4-difluoro-3-hydroxyoctyl)-5-hydroxy-3-(benzyl-2-oxo)-cyclopentyl]-5-heptenoate (LB-2) (50g) . After the addition, the reaction solution was heated to 25°C and stirred for 3-5 hours, and the reaction of the raw materials was complete. Slowly pour the reaction solution into 350g Na 2 S 2 o 3 and 160g NaHCO 3 Aqueous solution (1 L) of the solution, stirred for 15-30 minutes, added 500 mL of dichloromethane for extraction, separated the layers, back-extracted the aqueous layer with dichloromethane (500 mL), combined the dichloromethane layers, washed with water, and washed with brine. The organic phase was filtered with anhydrous sodium sulfate, and concentr...
Embodiment 2
[0031] Example 2: 7-[(2R,4aR,5R,7aR)-2-(1,1-difluoropentyl)-2-hydroxyl-6-oxooctahydrocyclopentadienopyran-5- Base] the preparation of heptanoic acid (lubiprostone)
[0032] (Z)-7-[(1R,2R,3R)-2-(4,4-difluoro-3-oxooctyl)-5-oxo-3-(benzyl-2-oxo) -Cyclopentyl]-5-heptenoic acid benzyl ester (LB-1) (42g) and ethyl acetate (420mL) were charged into a 1L medium-pressure reactor, nitrogen was replaced, and palladium carbon (4.2g) was added to replace hydrogen. 45°C, hydrogenation at 8atm for 6h, the reaction of raw materials was complete. Nitrogen was replaced, suction filtered, and the filtrate was concentrated under reduced pressure. Purified by silica gel column chromatography, eluting with n-hexane / ethyl acetate=3 / 1, collecting the separated liquid, concentrating under reduced pressure, and drying to obtain 7-[(2R,4aR,5R,7aR)-2-(1,1-di Fluoropentyl)-2-hydroxy-6-oxooctahydrocyclopentadienopyran-5-yl]heptanoic acid (lubiprostone) (26 g, yield 96%, HPLC purity 99.76%).
Embodiment 3
[0033] Example 3: (Z)-7-[(1R,2R,3R)-2-(4,4-difluoro-3-oxooctyl)-5-oxo-3-(benzyl-2- Preparation of benzyl oxo)-cyclopentyl]-5-heptenoate (LB-1)
[0034] Mix the Dess-Martin oxidant (100g) with dichloromethane (450mL), cool in an ice-water bath, control the internal temperature at 0-10°C, add (Z)-7-[(1R,2R,3R,5S)-2-( Benzyl 4,4-difluoro-3-hydroxyoctyl)-5-hydroxy-3-(benzyl-2-oxo)-cyclopentyl]-5-heptenoate (LB-2) (50g) . After the addition, the reaction solution was heated to 35°C and stirred for 5 hours, and the reaction of the raw materials was complete. Slowly pour the reaction solution into 350g Na 2 S 2 o 3 and 160g NaHCO 3 Aqueous solution (1 L) of the solution, stirred for 30 minutes, added 500 mL of dichloromethane for extraction, separated the layers, back-extracted the aqueous layer with dichloromethane (500 mL), combined the dichloromethane layers, washed with water, and washed with brine. The organic phase was filtered with anhydrous sodium sulfate, and concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com