Variant of tumor necrosis factor-related apoptosis-inducing ligand, and preparation method and application thereof
A technology of apoptosis-inducing ligand and tumor necrosis factor, which is applied in the field of genetic engineering and achieves good clinical application prospects and excellent curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 Preparation of TRAIL variant of the present invention
[0043] 1. Design and gene cloning of TRAIL variants
[0044] 1) Design of TRAIL variants
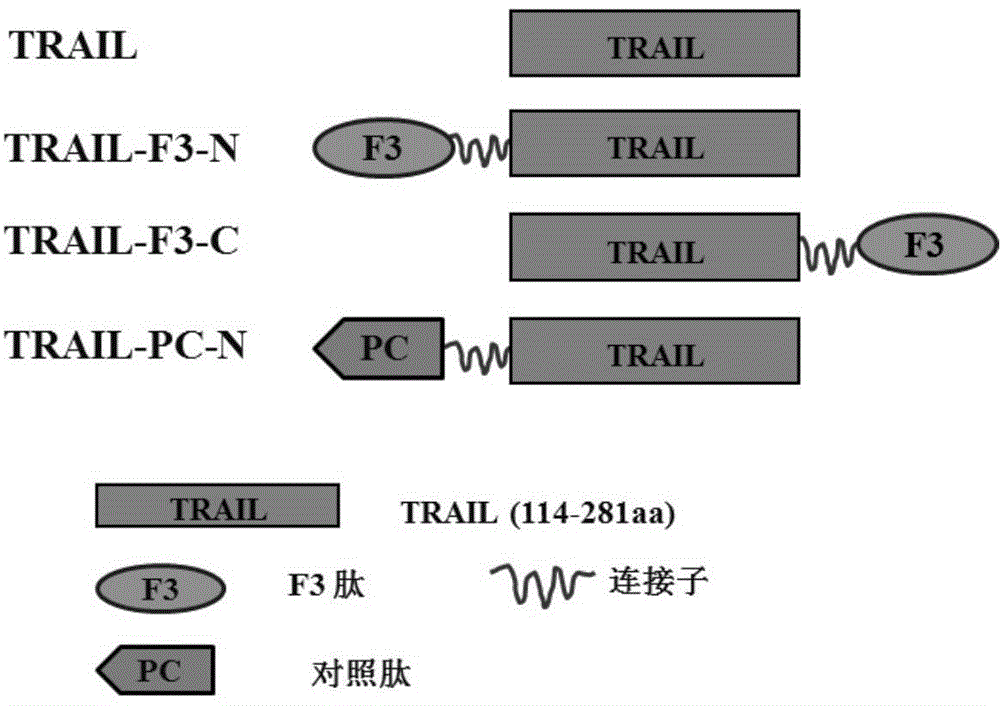
[0045] The F3 peptide consists of 31 amino acids. TRAIL is a fragment that intercepts the amino acid composition of human TRAIL114-281. F3 can be connected to the N-terminal or C-terminal of TRAIL, and a flexible linker is added between the two fragments, such as (G4S) 3 Wait. Such as figure 1 As shown, the fusion proteins formed by linking F3 at the N-terminus or C-terminus of TRAIL were named TRAIL-F3-N and TRAIL-F3-C, respectively. At the same time, the control peptide PC was selected and connected to the N-terminus of TRAIL to construct TRAIL-PC-N as a control.
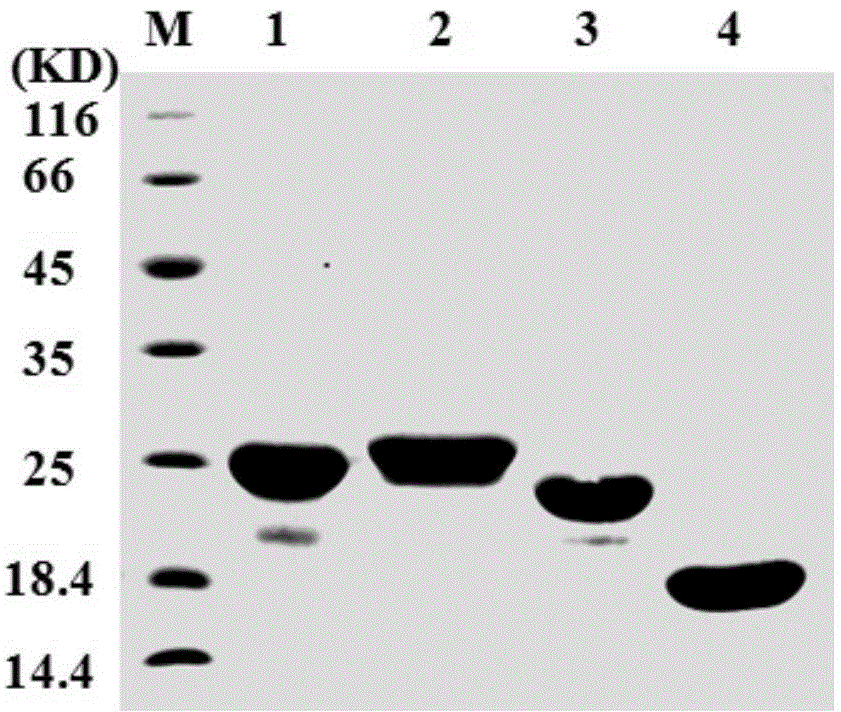
[0046] Using nucleic acid analysis software, the coding genes of each fragment were spliced to obtain the coding genes of TRAIL variant proteins TRAIL-F3-N, TRAIL-F3-C and TRAIL-PC-N, and then submitted to Nanjing GenScript Company for artifi...
experiment example 1
[0060] Experimental example 1 Effect of the covalent linking method of functional fragments of TRAIL variants on the activity of the variants
[0061] 1. Experimental method
[0062] The cell-killing activity of the TRAIL variant protein was detected by an in vitro cytotoxicity test model.
[0063] Experiments were carried out with human liver cancer cell line SMMC-7721 and human lung cancer cell line A549: the cells were placed in RPMI 1640 containing 10% calf serum, 2mM L-glutamine, 100μg / ml streptomycin and 100U / ml penicillin. 37°C, 5% CO 2 cultivated under conditions. Will 1×10 4 Cells were seeded in a 96-well plate and adhered to the wall overnight, and then the medium was replaced with 1640 medium containing 2% calf serum, and proteins of different concentrations were added at the same time. After acting overnight, CCK-8 solution was added and reacted for 2-4 hours. Afterwards, the absorbance at 495 nm was measured with a microplate reader. The cell survival rate of...
experiment example 2
[0075] Experimental Example 2 Detection of the Selective Killing Properties of Variant Proteins on Tumor Cells
[0076] 1. Experimental method
[0077] The in vitro cytotoxicity test model was used to compare the killing activity of TRAIL variant protein on tumor cells and normal cells, so as to determine its cell killing selectivity. Will 1×10 4 Cells (100 μl) were inoculated in a 96-well plate and adhered to the wall overnight, and then the culture medium was replaced with 1640 medium containing 2% calf serum, and the TRAIL and TRAIL variant proteins obtained in different concentrations of Example 1 were added simultaneously to act overnight Afterwards, 10 μl of CCK-8 solution was added, and the absorbance at 495 nm was measured with a microplate reader after reacting for 2-4 hours. The cell survival rate of the protein treatment group was calculated as the cell survival rate of the non-protein treatment group as 100%.
[0078] 2. Experimental results
[0079] The result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com