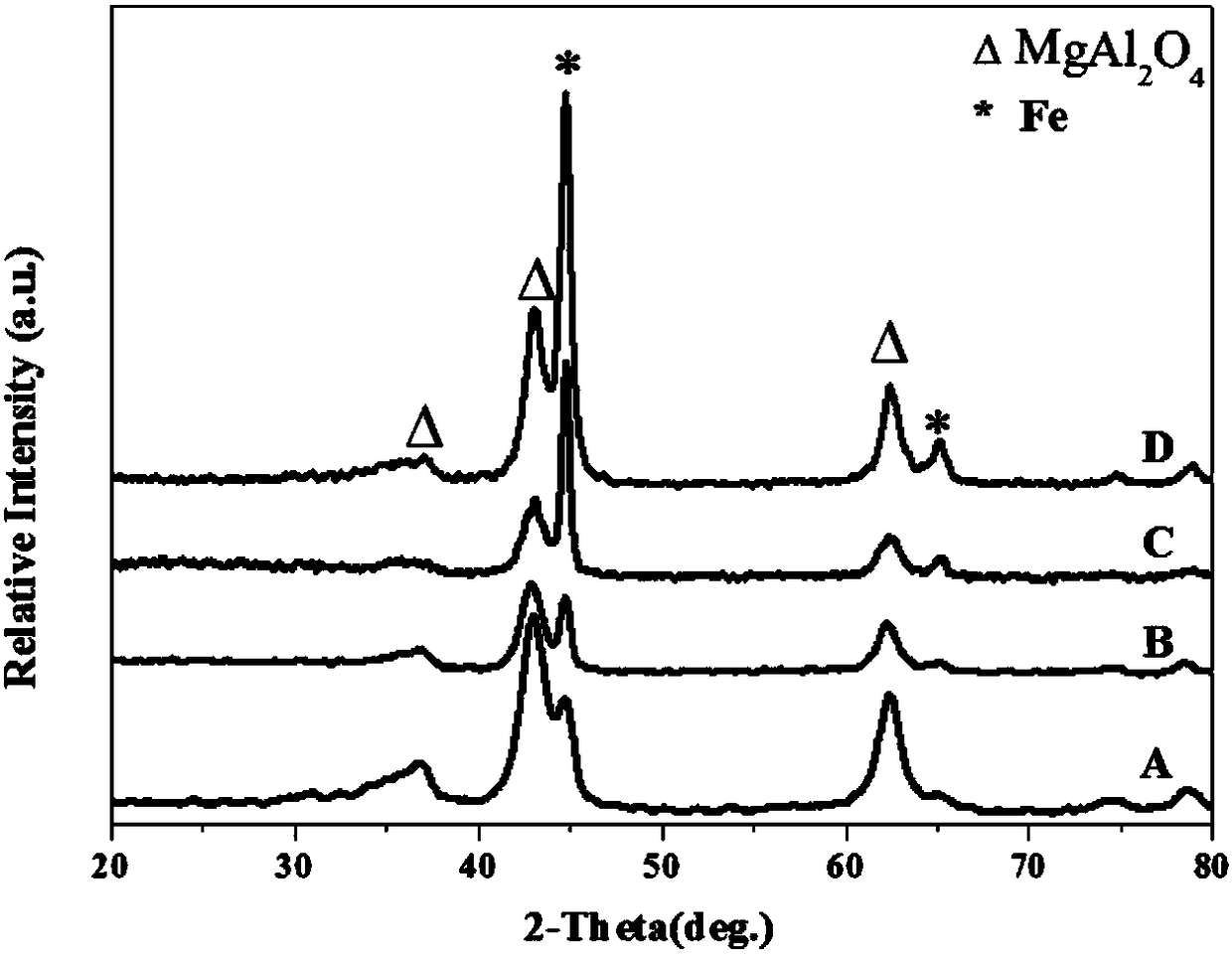
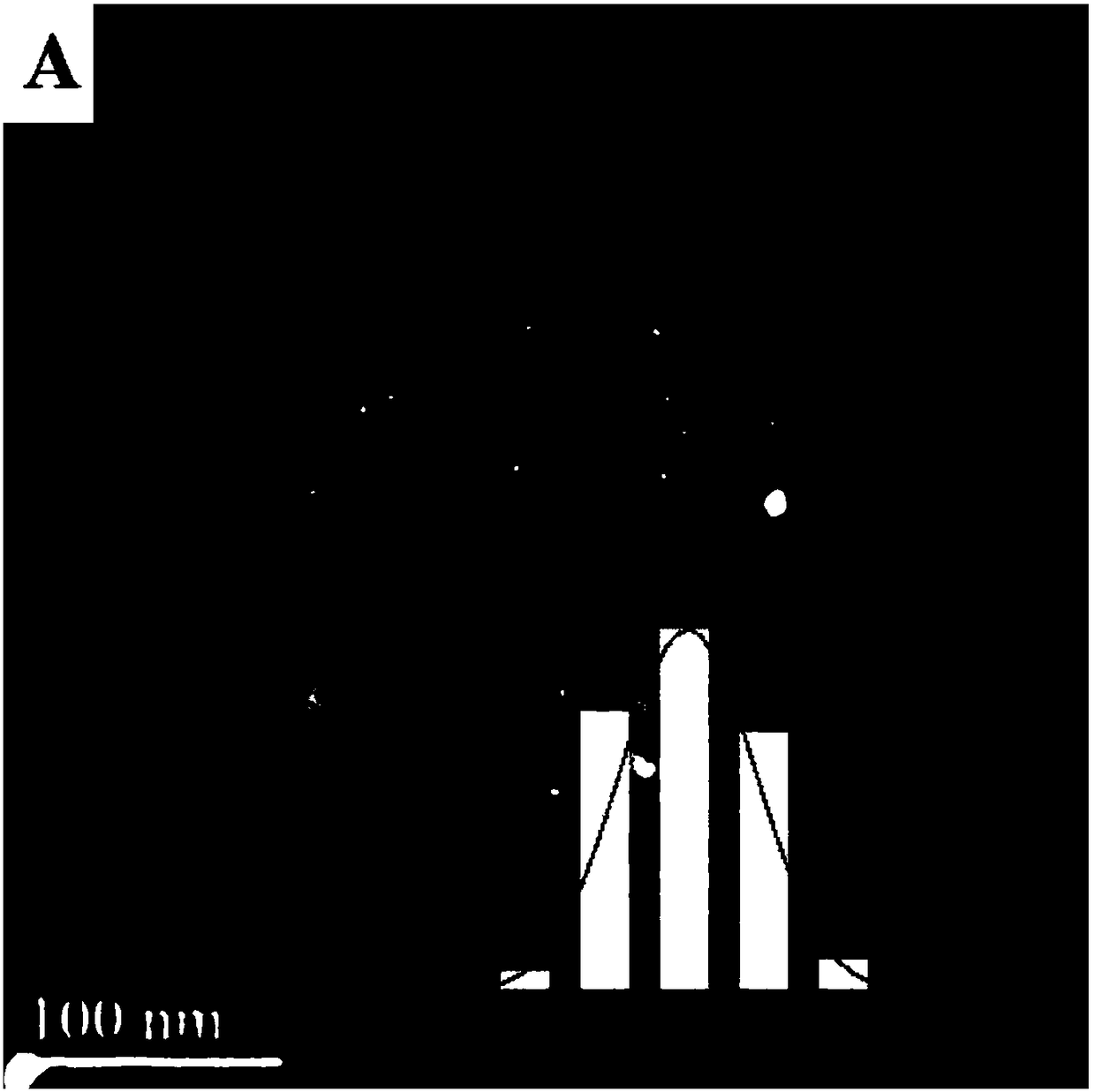
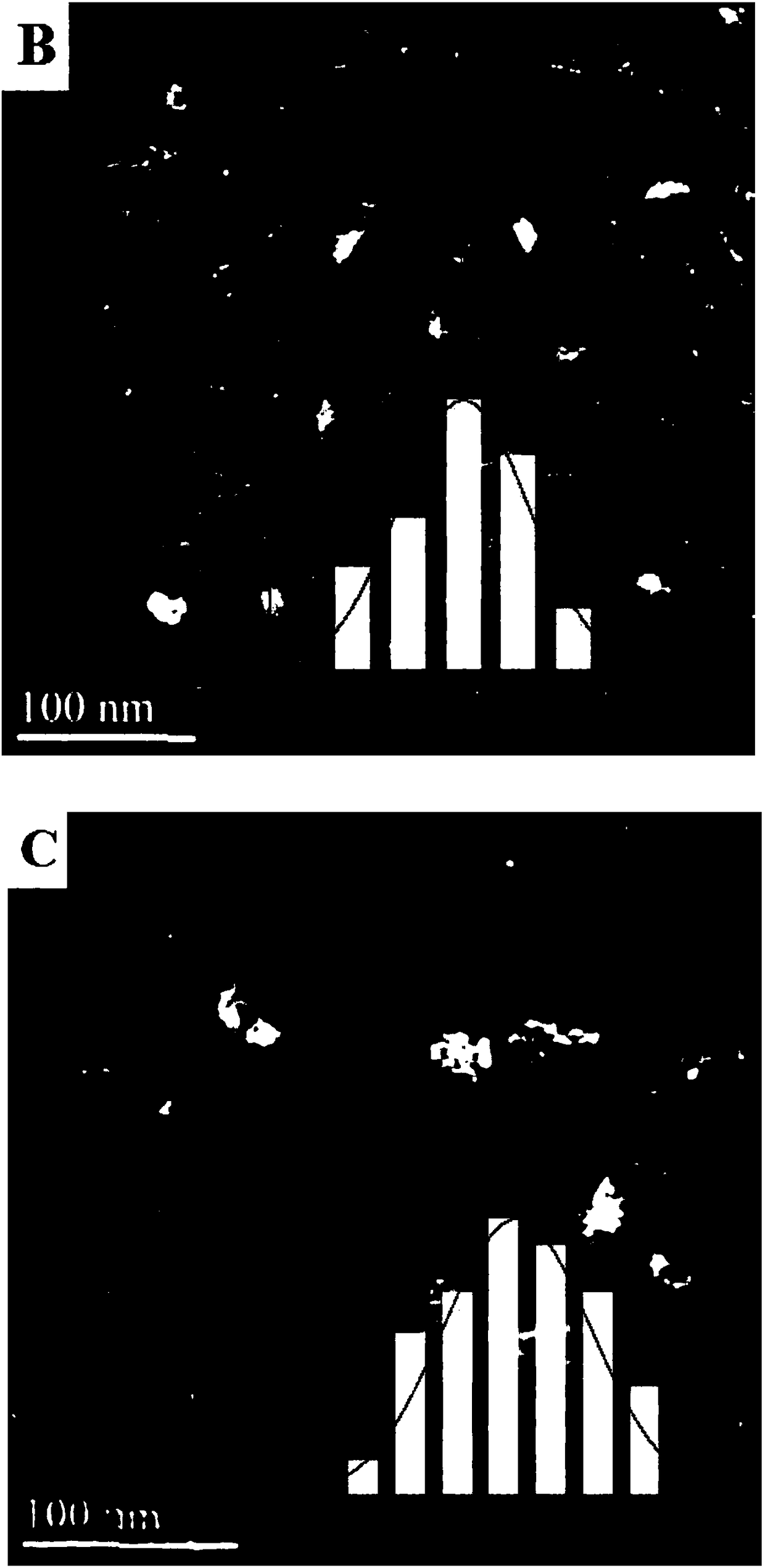
A Fe-based catalyst with higher CO hydrogenation reaction activity and low olefin yield and its application
A low-carbon olefin and catalyst technology, which is applied in the field of CO catalytic hydrogenation to achieve the effects of easy preparation, enhanced adsorption and dissociation ability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step A: take by weighing 8.5467g Mg (NO 3) 2 ·6H 2 O, 1.2504g Al(NO 3 ) 3 9H 2 O, 5.3867g Fe(NO 3 ) 3 9H 2 O was put into a beaker and dissolved in 250mL deionized water; another 3.533g Na 2 CO 3 and 3.2g NaOH were put into another beaker, and 250mL deionized water was also added for dissolution. The two solutions were slowly dropped into a 1000ml four-neck flask at the same time, and the pH of the solution was strictly controlled to keep it constant at 9.5. Then the temperature was raised to 95° C. and kept for 20 hours for crystallization. After suction filtration, washing, drying and grinding, MgAlFe-CO can be obtained 3 2- - LDHs samples.
[0028] Step B: Take the partially ground MgAlFe-CO 3 2- - LDHs powder is put into the porcelain boat, and then it is placed near the thermocouple of the tube resistance furnace, and then the inside of the tube is evacuated. After observing that the pressure in the tube does not change, the H 2 to return to normal...
Embodiment 2
[0031] Step A: take by weighing 8.5467g Mg (NO 3 ) 2 ·6H 2 O, 1.2504g Al(NO 3 ) 3 9H 2 O, 5.3867g Fe(NO 3 ) 3 9H 2 O was put into a beaker and dissolved in 250mL deionized water; another 3.533g Na 2 CO 3 and 3.2g NaOH were put into another beaker, and 250mL deionized water was also added for dissolution. The two solutions were slowly dropped into a 1000ml four-neck flask at the same time, and the pH of the solution was strictly controlled to keep it constant at 9.5. Then the temperature was raised to 95° C. and kept for 20 hours for crystallization. After suction filtration, washing, drying and grinding, MgAlFe-CO can be obtained 3 2- - LDHs samples.
[0032] Step B: Take the partially ground MgAlFe-CO 3 2- - LDHs powder is put into the porcelain boat, and then it is placed near the thermocouple of the tube resistance furnace, and then the inside of the tube is evacuated. After observing that the pressure in the tube does not change, the H 2 to return to norma...
Embodiment 3
[0035] Step A: take by weighing 8.5467g Mg (NO 3 ) 2 ·6H 2 O, 1.2504g Al(NO 3 ) 3 9H 2 O, 5.3867g Fe(NO 3 ) 3 9H 2 O was put into a beaker and dissolved in 250mL deionized water; another 3.533g Na 2 CO 3 and 3.2g NaOH were put into another beaker, and 250mL deionized water was also added for dissolution. The two solutions were slowly dropped into a 1000ml four-neck flask at the same time, and the pH of the solution was strictly controlled to keep it constant at 9.5. Then the temperature was raised to 95° C. and kept for 20 hours for crystallization. After suction filtration, washing, drying and grinding, MgAlFe-CO can be obtained 3 2- - LDHs samples.
[0036] Step B: Take the partially ground MgAlFe-CO 3 2- - LDHs powder is put into the porcelain boat, and then it is placed near the thermocouple of the tube resistance furnace, and then the inside of the tube is evacuated. After observing that the pressure in the tube does not change, the H 2 to return to norma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com