Catalyst used for hydroformylation of olefin, and preparation method and application thereof
An alkene hydroformyl and catalyst technology, which is applied in the field of catalyst preparation and application, can solve few problems and achieve the effects of good activity, increased selectivity and good flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
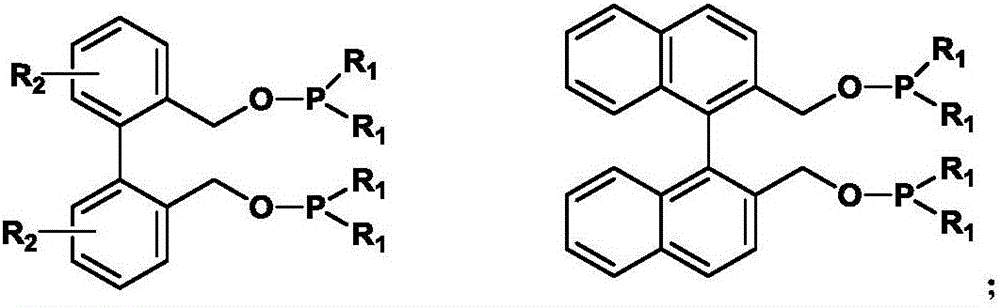
[0034] Synthesis of 2,2-dimethylol-1,1’-biphenyl skeleton:
[0035]
[0036] 1,1’-biphenyl-2,2’-dicarboxylic acid Depend on Obtained by oxidation of phenanthrene: Add phenanthrene (8.9g, 50mmol) into a 150ml three-neck flask, then add glacial acetic acid (100ml) to dissolve the solid, raise the temperature to 85°C, slowly add H 2 o 2 (30%, 34.5ml) solution, 40min was added dropwise; After the end of the dropwise addition, the reaction temperature was controlled to be 80°C, and part of the raw material phenanthrene may be precipitated; reflux and stirred for 4 hours, cooled; half of the volume of the solvent was removed under reduced pressure, statically Placed, a light yellow solid precipitated out. After filtration, the crude product was obtained as a pale yellow solid; the weight was 10.9 g, and the yield was 90.0%.
[0037] 1 H NMR (400MHz, CD 3 OD), δ=8.00(d, J=8.0Hz, 2H), 7.55(t, J=8.0Hz, 2H), 7.44(t, J=8.0Hz, 2H), 7.19(t, J=8.0Hz, 2H)
[0038] Add NaBH to th...
Embodiment 2
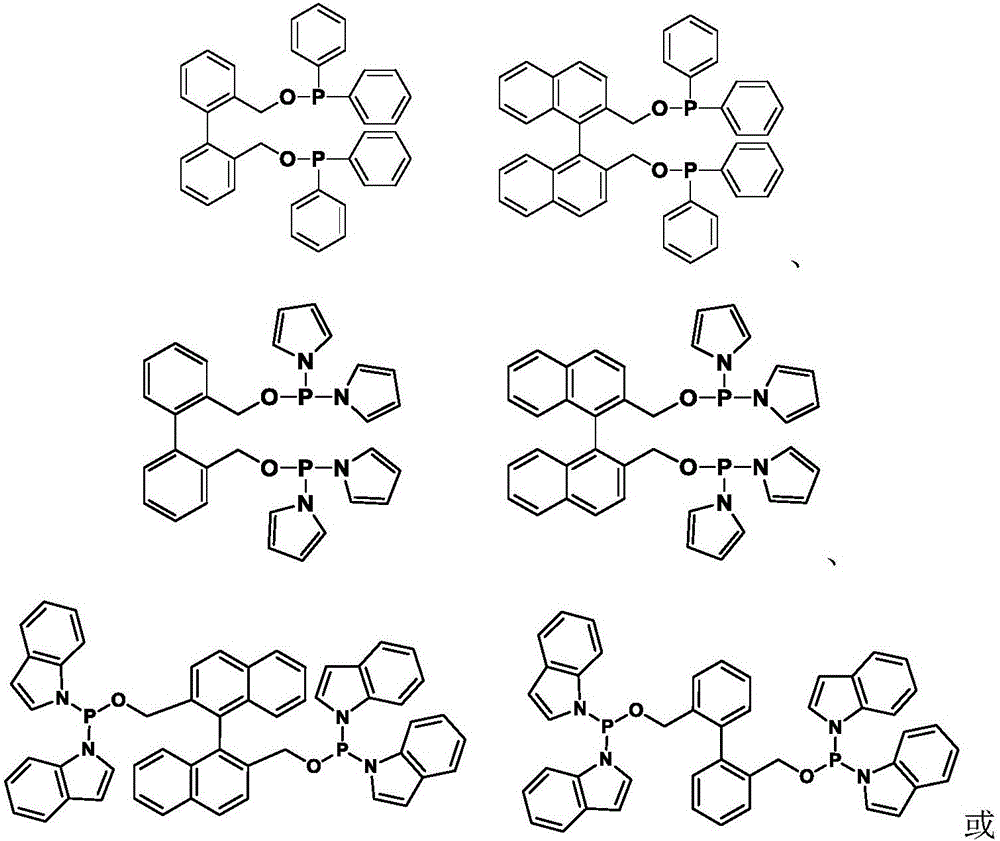
[0041] Synthesis of bidentate phosphonite phosphine ligands:
[0042]
[0043] In a 100ml three-neck bottle, add (0.7g, 3.3mmol) and 4-dimethylaminopyridine (80mg, 0.65mmol) were dissolved in 50ml of tetrahydrofuran solution after anhydrous and oxygen-free treatment, and diphenylphosphorous chloride (1.30 ml, 7.26mmol), at -15°C, under the protection of N2, a tetrahydrofuran (15ml) solution of triethylamine (2ml) was added dropwise in 30 minutes; after the dropwise addition was completed, the reaction was continued at room temperature for 2 hours; minutes, then filtered under N2 atmosphere to remove triethylamine hydrochloride, and the solvent tetrahydrofuran was removed under reduced pressure; a pale yellow oil was obtained; subsequently extracted with treated n-hexane; n-hexane was removed under reduced pressure to obtain a colorless oil. Weight 0.86g, yield: 45.0%.
[0044] 1H NMR (400MHz, d6-DMSO): δ = 7.55 (d, J = 7.4Hz, 2H), 7.44–7.39 (m, 2H), 7.37–7.26 (m, 22H), ...
Embodiment 3
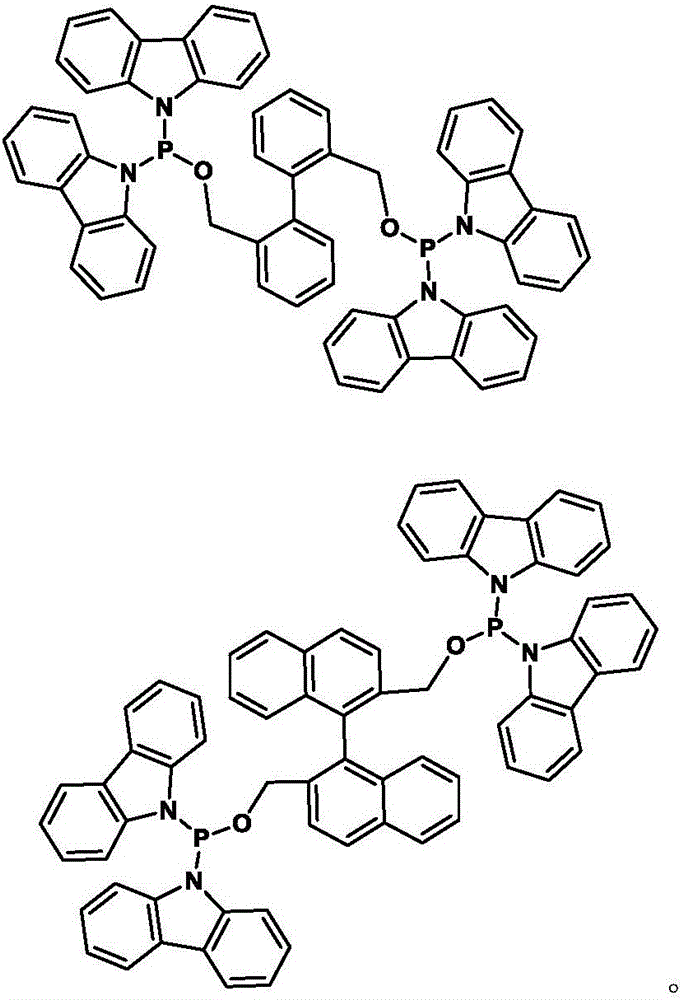
[0046] Synthesis of bidentate phosphonamidophosphine ligands:
[0047]
[0048] Dipyrrole phosphorus chloride Synthesized from pyrrole and phosphorus trichloride: in N 2 Or under the protection of Ar gas atmosphere, add anhydrous tetrahydrofuran (120ml) and phosphorus trichloride (5.3ml, 0.06mol) to the 250ml three-necked flask, and add pyrrole (8.4ml, 0.12mol) and trichloride dropwise under ice bath conditions. Ethylamine (25.0ml, 0.18mol) in anhydrous tetrahydrofuran (30ml), slowly added dropwise (about 1h), then warmed up to room temperature and stirred overnight; stop the reaction and let stand for 20 minutes, filter under N2 atmosphere to remove triethylamine Hydrochloride, most of the solvent tetrahydrofuran was removed under reduced pressure, and the residue was distilled under reduced pressure to collect the product at 80°C (0.1mmHg). The product is a colorless oil. Weight 5.3g, yield 45.0%.
[0049] 31 P NMR (166MHz, CDCl3): δ=103.63.
[0050] Under N2 or Ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com