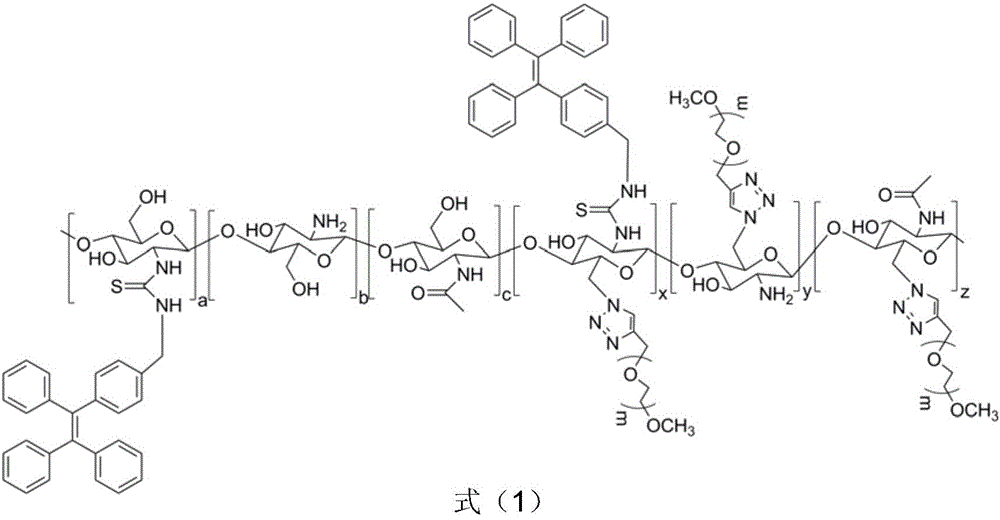
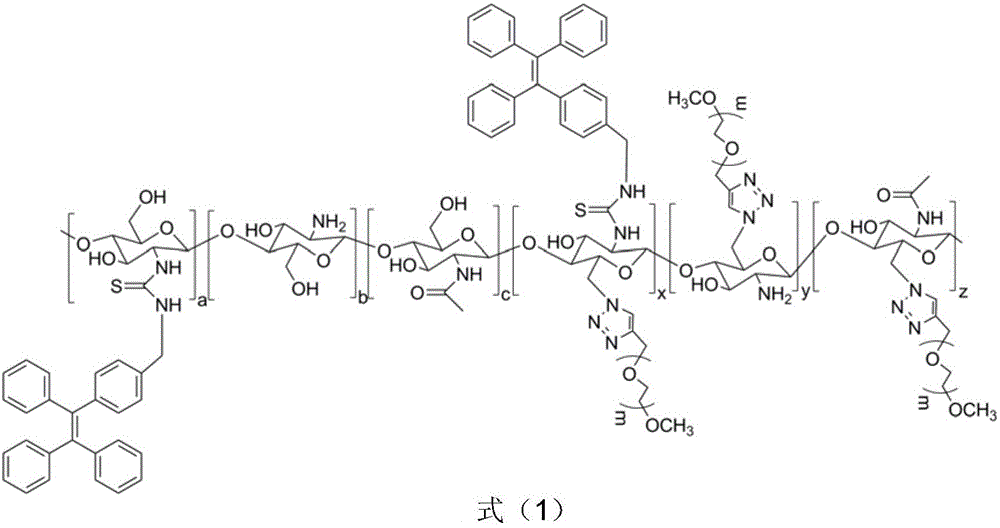
Chitosan-based fluorescence probe suitable for long circulation of blood and preparing method thereof
A fluorescent probe and long-circulation technology, applied in the long-circulation blood chitosan-based fluorescent probe and its preparation field, can solve the problems of cytotoxicity and other problems, achieve high sensitivity, no drift of fluorescence spectrum, and good imaging effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) in N 2 Under the atmosphere, add 1g viscosity-average molecular weight in the there-necked flask to be 10,000, the degree of deacetylation is 60% chitosan (CS) and 2.30g phthalic anhydride, add 40ml N,N-dimethylformamide ( DMF), reacted at 130°C for 5 hours, poured it into ice water to precipitate, extracted the precipitate with methanol for 24 hours after suction filtration, and dried it in vacuum at 40°C to obtain N,O-phthalylated chitosan sugar.
[0023] 2) Add 1.2g of N,O-phthalylated chitosan to a mixed solvent of 100ml N,N-dimethylformamide and water, the volume ratio of N,N-dimethylformamide to water The ratio is 95:5, heated to 120°C in a nitrogen atmosphere, and after being fully stirred, the product is placed in ice water for precipitation, filtered with suction, extracted with methanol for 24 hours, and dried under vacuum at 40°C to obtain N-phthaloyl Chitosan;
[0024] 3) 0.5g of N-phthalylated chitosan was dissolved in 30ml of N,N-dimethylacetamide so...
Embodiment 2
[0034] 1) Under N2 atmosphere, add 1g CS (viscosity average molecular weight is 10,000, deacetylation degree is 60%) and 2.30g phthalic anhydride to the there-necked flask, add 40ml DMF, react at 130°C for 5 hours, Pour it into ice water to precipitate, and after suction filtration, extract the precipitate with methanol for 24 hours, and dry it in vacuum at 40°C to obtain N,O-phthalylated chitosan;
[0035] 2) Add 1.2g of N,O-phthalylated chitosan to a mixed solvent of 100ml N,N-dimethylformamide and water, the volume ratio of N,N-dimethylformamide to water The ratio is 95:5, heated to 120°C in a nitrogen atmosphere, and after being fully stirred, the product is placed in ice water for precipitation, filtered with suction, extracted with methanol for 24 hours, and dried under vacuum at 40°C to obtain N-phthaloyl Chitosan;
[0036] 3) 0.5g of N-phthalylated chitosan was dissolved in 30ml of N,N-dimethylacetamide solution with a mass concentration of 5% lithium chloride, pre-co...
Embodiment 3
[0045] 1) in N 2 Under atmosphere, add 1g CS (viscosity-average molecular weight: 200,000, degree of deacetylation: 70%) and 2.30g phthalic anhydride to the three-necked flask, add 40ml DMF, react at 130°C for 6 hours, pour it into Precipitate in ice water, extract the precipitate with methanol for 24 hours after suction filtration, and dry under vacuum at 40°C to obtain N,O-phthalylated chitosan;
[0046] 2) Add 1.2g of N,O-phthalylated chitosan to a mixed solvent of 100ml N,N-dimethylformamide and water, the volume ratio of N,N-dimethylformamide to water The ratio is 95:5, heated to 120°C in a nitrogen atmosphere, and after being fully stirred, the product is placed in ice water for precipitation, filtered with suction, extracted with methanol for 24 hours, and dried under vacuum at 40°C to obtain N-phthaloyl Chitosan;
[0047] 3) 0.5g of N-phthalylated chitosan was dissolved in 30ml of N,N-dimethylacetamide solution with a mass concentration of 5% lithium chloride, pre-co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com