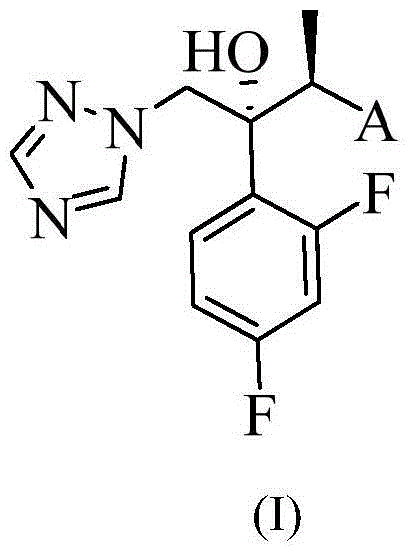
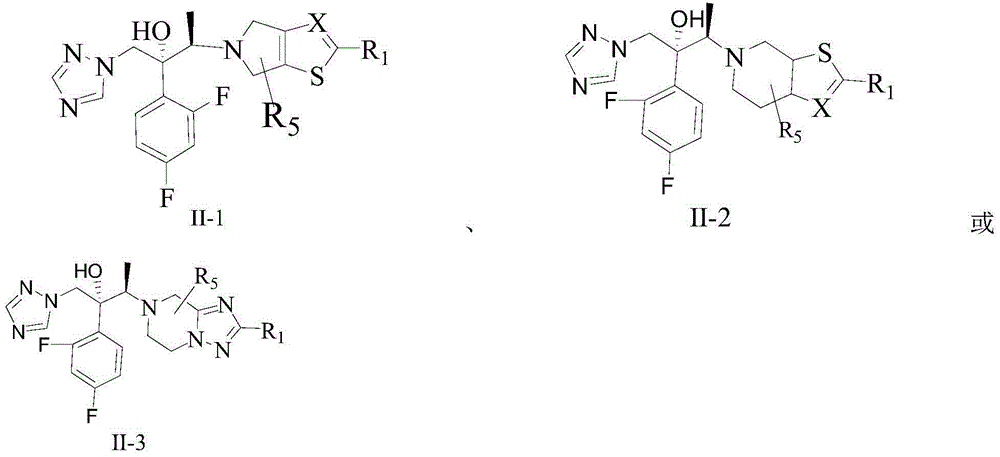
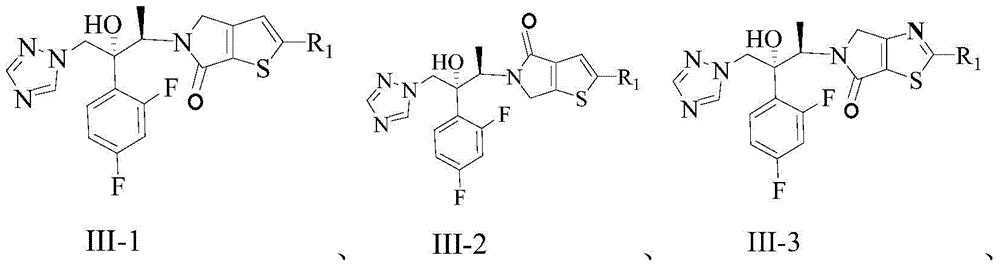
Triazole compound, pharmaceutical composition, preparation method and uses thereof
A compound, the technology of triazole, which is applied in the field of preparation of antifungal drugs, can solve the problems of low oral bioavailability, low bioavailability, and the curative effect is greatly affected by food, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: 2-bromo-5-((2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-4-(1H-1,2,4-triazole-1- yl)but-2-yl)-4,5-dihydrothieno[3,2-c]pyrrol-6-one (compound 1)
[0106] (a) 5-bromo-3-((((2R,3R)-3-(2,4-difluorophenyl)-3-hydroxyl-4-(1H-1,2,4-triazole-1 -yl)but-2-yl)amino)methyl)thiophene-2-carboxylic acid methyl ester
[0107]
[0108] Dissolve compound 2A [EP332387A1, 1989] (3.21g, 12.00mmol), compound 1-1 [WO2007 / 124546A1, 2007] (4.14g, 13.18mmol) in 60.0ml of acetonitrile, and add After the addition of K2CO3 (1.99g, 14.38mmol), slowly rise to room temperature, stir overnight, concentrate the solvent under reduced pressure, add ethyl acetate and water to extract, the organic layer is washed with saturated brine, dried, filtered, concentrated, column layer Analysis (petroleum ether: ethyl acetate = 4:1 to 2:1) gave an orange-yellow oil, 4.86 g in total, with a yield of 80.7%.
[0109] 1 H NMR (400MHz, CDCl 3 )δ7.89(s,1H),7.75(s,1H),7.36(dd,J=15.6,8.8Hz,1H),7.17(s,1H),6.73(...
Embodiment 2
[0118] Example 2: 5-((2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-4-(1H-1,2,4-triazol-1-yl)butan- Preparation of 2-yl)-2-(pyridin-3-yl)-4,5-dihydrothieno[3,2-c]pyrrol-6-one (Compound 2)
[0119]
[0120] Compound 1 (99.00mg, 0.21mmol), 3-pyridine borate pinadate (60.00mg, 0.28mmol), tetrakis (triphenylphosphine) palladium (30.00mg, 0.02mmol), cesium carbonate (138.80mg, 0.43mmol ) was dissolved in an aqueous solution of dioxane (15.0ml, 2:1), and reacted at 80°C for 12h under the protection of argon. Concentrate to dryness, add ethyl acetate and water solution, extract the aqueous layer with ethyl acetate, combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, column chromatography (dichloromethane: Methanol=200:1~100:1), 70.00 mg of white solid compound 2 was obtained, and the yield was 71.2%.
[0121] 1 H NMR (500MHz, CDCl 3 )δ8.91(s,1H),8.61(d,J=4.2Hz,1H),7.91(d,J=7.9Hz,1H),7.87(s,1H),7.72(s,1...
Embodiment 3
[0122] Example 3: 5-((2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-4-(1H-1,2,4-triazol-1-yl)butan- Preparation of 2-yl)-2-(pyridin-4-yl)-4,5-dihydrothieno[3,2-c]pyrrol-6-one (Compound 3)
[0123]
[0124] Compound 1 (99.00mg, 0.21mmol), pyridine-4-boronic acid (34.40mg, 0.28mmol), tetrakis (triphenylphosphine) palladium (30.00mg, 0.02mmol), cesium carbonate (138.80mg, 0.43mmol), Prepared according to a method similar to Example 2, 64.70 mg of white solid compound 3 was obtained, with a yield of 65.6%.
[0125] 1 H NMR (400MHz, CDCl 3)δ8.67(d, J=5.5Hz, 2H), 7.85(s, 1H), 7.72(s, 1H), 7.52(d, J=6.1Hz, 2H), 7.48(s, 1H), 7.43( m,1H),6.83–6.74(m,2H),5.20(d,J=13.6Hz,1H),4.98(d,J=14.3Hz,2H),4.56(d,J=14.3Hz,1H), 4.30(d,J=14.0Hz,1H),1.15(d,J=7.1Hz,3H).MS(EI)m / z:467(M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com