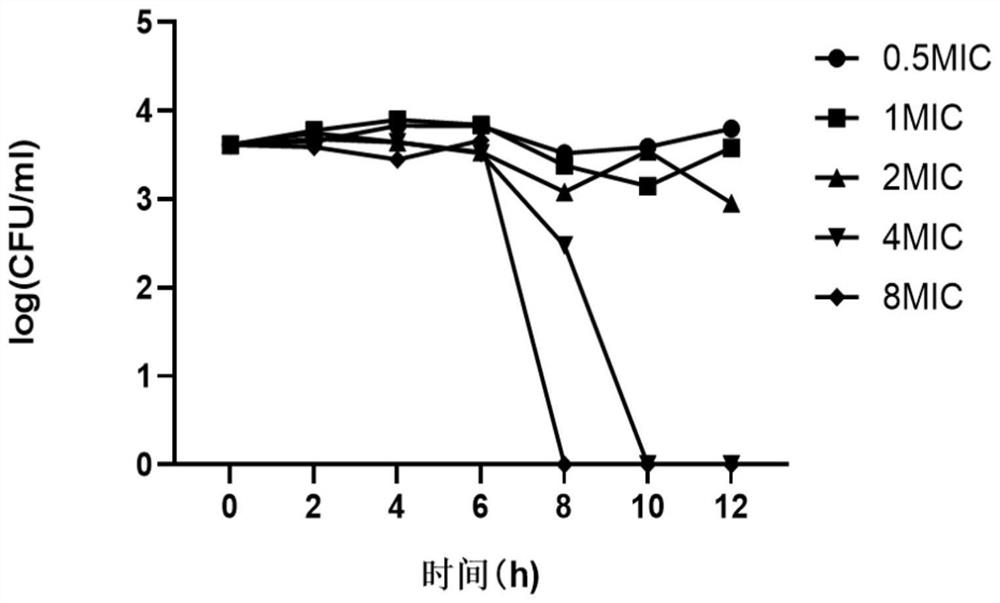
Application of allicin in preparation of anti-saccharomycetes drugs
A technology of allicin and yeast, applied in the field of medicine, can solve problems such as no report on the application of allicin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of allicin and product determination.
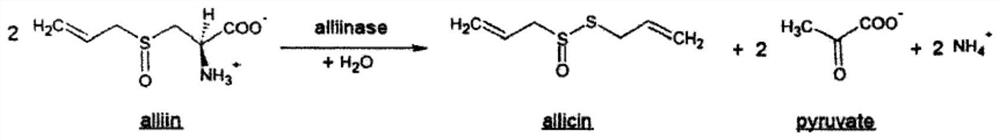
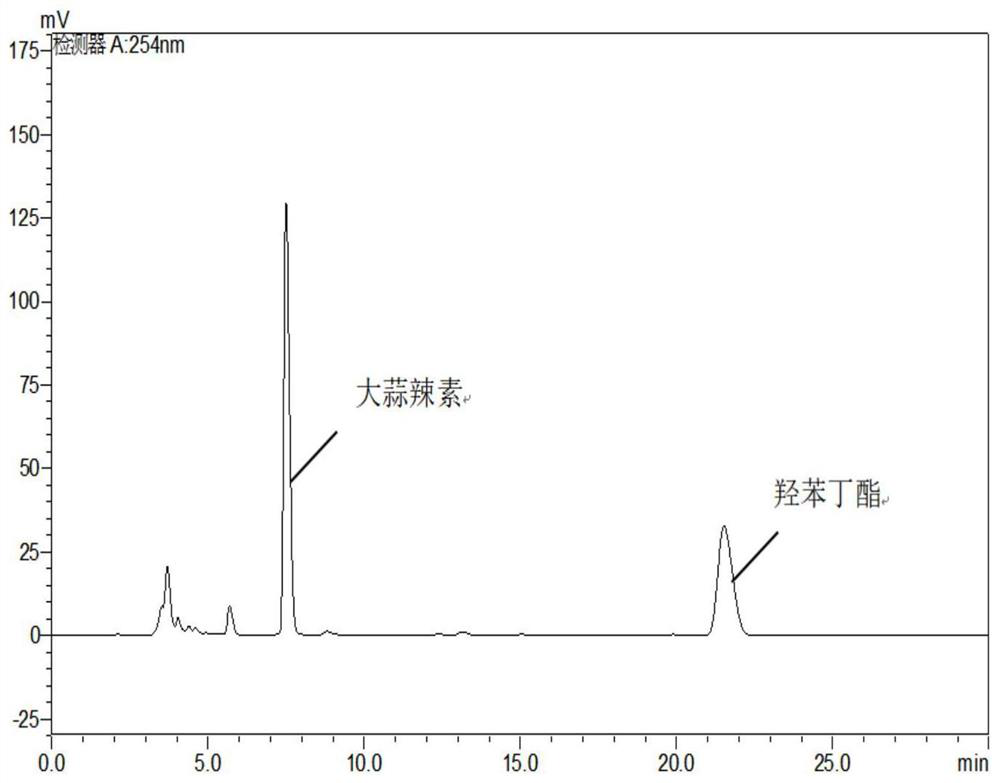
[0037] Alliin-alliinase Weigh 290mg and 580mg of alliinin according to the mass ratio of 1:2, put them in 10ml measuring bottles, add water to dissolve and dilute to the scale, transfer the allininase solution to a 50ml conical flask with a stopper , quickly add the alliin solution to the allinase solution, mix well, and react at room temperature for 30 minutes. Precisely pipette 0.5ml of the reaction solution into a 25ml measuring bottle, add 15ml of methanol, add 1.0ml of internal standard solution, dilute to the mark with 1% formic acid solution, shake well, filter, and obtain. Precisely draw 20 μl of the test solution, inject it into the high-performance liquid chromatograph, and record the chromatogram (see figure 2 ) to calculate the amount of allicin produced (see Table 1).
[0038] Table 1 Allicin Content Determination Results
[0039]
[0040] Note: A x Represents the peak area of the test solution, A ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com