Eimeria tenella microneme protein-2 mutant EtMIC2-1130 of chickens
A technology of Eimeria and mutants, applied in the field of genetic engineering and genetic engineering, can solve the problems of high production cost, inability to fundamentally improve antigen immunogenicity, safety of live vaccines, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
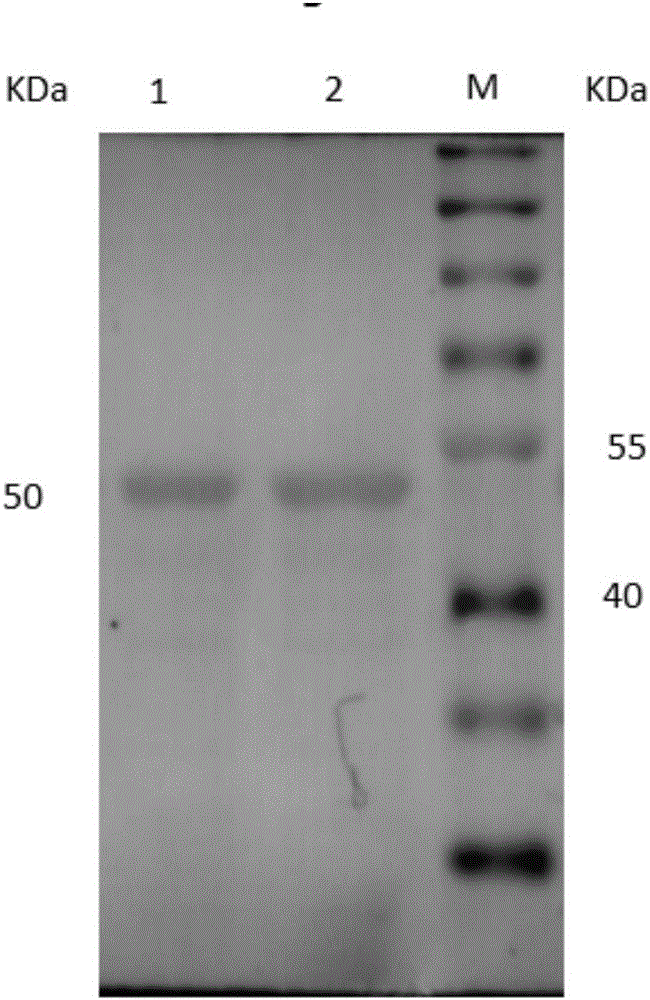
[0034] The acquisition of embodiment 1 mutant EtMIC2-1130
[0035] First, according to the EtMIC2 nucleotide sequence published by GenBank (GenBank: KC333870.1) artificially synthesized natural EtMIC2 sequence, the obtained EtMIC2 nucleotide sequence, its nucleotide sequence is shown in Seq ID No: 3, and then it was compared with pEASY-T1 vector ligation modeling EtMIC2-T1;
[0036]Then use EtMIC2-T1 as a template, use EtMIC2-951-F and EtMIC2-951-R as primers, and use Diversify PCR Random Mutagenes Kit (Clontech, USA) to perform error-prone PCR amplification of EtMIC2 mutants to obtain error-prone PCR products; The primers used are shown in Table 1:
[0037] Table 1. The mutant EtMIC2-1130 specific primers
[0038]
Embodiment 2
[0039] The preparation of mutant described in embodiment 2
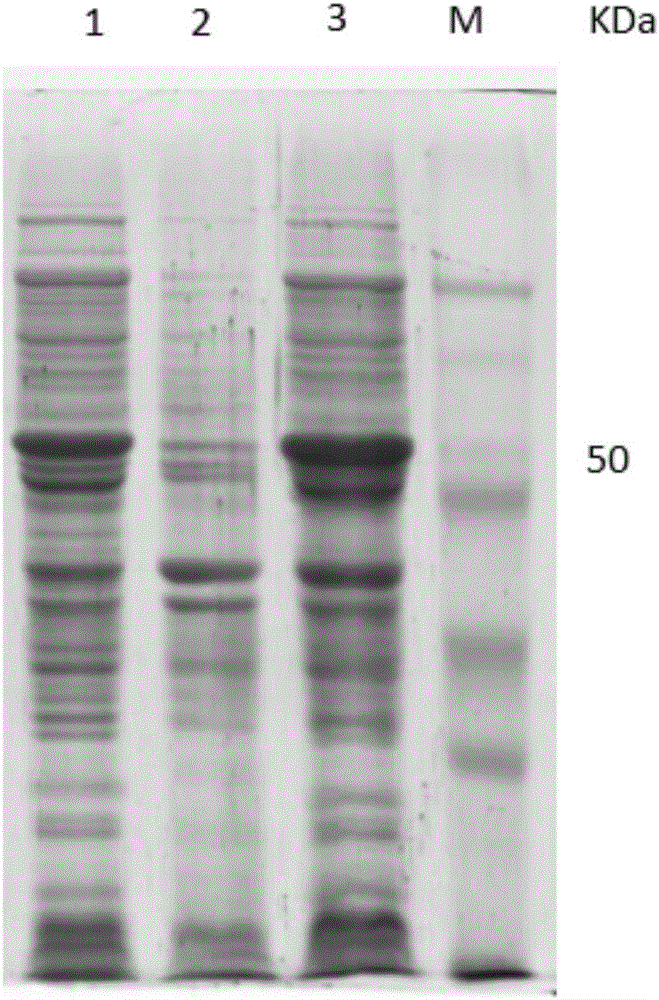
[0040] The error-prone PCR product obtained in Example 1 was connected to the pEASY-T1 vector without purification, and then transferred into Top 10 competent cells, coated with ampicillin LB agar plate, and cultivated in a 37°C incubator until single Clonal colonies increased to 10 7 So far, construct the EtMIC2 gene mutation library;
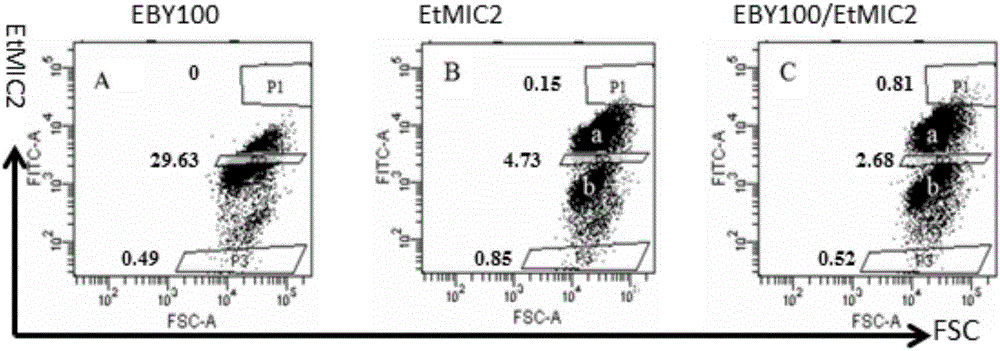
[0041] The above-mentioned recombinant plasmid containing the gene mutation library and the pCTCON2 plasmid were double-digested with Nhe I and Sal I, and the double-digested gene mutation library was connected to the pCTCON2 vector and then transfected into Saccharomyces cerevisiae EBY100 (purchased from Invitrogen) to obtain a recombinant Strain EBY100 / EtMIC2, positive colonies identified by PCR (primers used for design are shown in Table 2); take the strain EBY100 containing the recombinant plasmid, spread it on the SD-CAA solid medium in a 30°C incubator, and cultivate it for 48...
Embodiment 3
[0049] Example 3 Determination of the immune protection effect of mutant EtMIC2-1130
[0050] The 1-day-old chicks were weighed, and the chickens with large weight differences were removed. A total of 100 chicks were divided into 4 groups, namely PBS-I (non-immune and non-infection group), PBS-II (non-immune infection group), natural protein EtMIC2, mutant EtMIC2 immunization group, 25 chicks in each group. Breed in hood. At the age of 7 days, chickens in each group were subcutaneously immunized with a final concentration of 0.5 mg / mL of protein or PBS 100 μL in Freund's complete adjuvant emulsification, and at the age of 14 days, they were boosted with Freund's incomplete adjuvant Emulsify 100 μL of protein or PBS corresponding to a final concentration of 0.5 mg / mL. The 21-day-old chicks were orally inoculated with 30,000 sporulated oocysts except the PBS-I group. The immune protection effect of the mutant protein was determined based on the body weight growth of immunized...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com